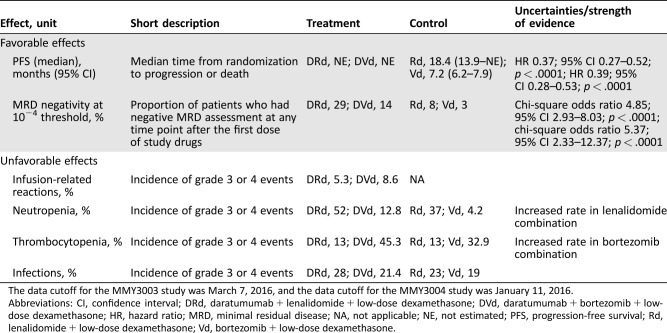
Table 2. Key favorable and unfavorable effects for DRd versus Rd and for DVd versus Vd.
The data cutoff for the MMY3003 study was March 7, 2016, and the data cutoff for the MMY3004 study was January 11, 2016.
Abbreviations: CI, confidence interval; DRd, daratumumab + lenalidomide + low‐dose dexamethasone; DVd, daratumumab + bortezomib + low‐dose dexamethasone; HR, hazard ratio; MRD, minimal residual disease; NA, not applicable; NE, not estimated; PFS, progression‐free survival; Rd, lenalidomide + low‐dose dexamethasone; Vd, bortezomib + low‐dose dexamethasone.