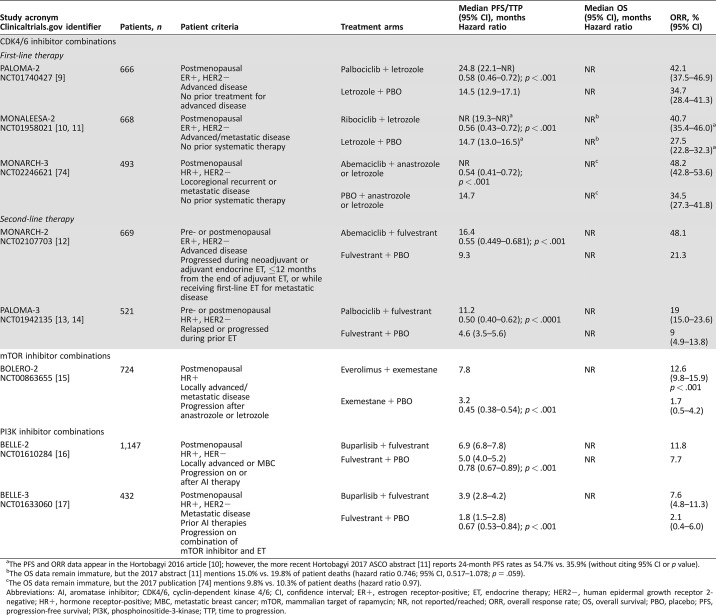
Table 1. Completed phase III studies of endocrine‐based combination therapies for advanced or metastatic breast cancer.
The PFS and ORR data appear in the Hortobagyi 2016 article [10]; however, the more recent Hortobagyi 2017 ASCO abstract [11] reports 24‐month PFS rates as 54.7% vs. 35.9% (without citing 95% CI or p value).
The OS data remain immature, but the 2017 abstract [11] mentions 15.0% vs. 19.8% of patient deaths (hazard ratio 0.746; 95% CI, 0.517–1.078; p = .059).
The OS data remain immature, but the 2017 publication [74] mentions 9.8% vs. 10.3% of patient deaths (hazard ratio 0.97).
Abbreviations: AI, aromatase inhibitor; CDK4/6, cyclin‐dependent kinase 4/6; CI, confidence interval; ER+, estrogen receptor‐positive; ET, endocrine therapy; HER2−, human epidermal growth receptor 2‐negative; HR+, hormone receptor‐positive; MBC, metastatic breast cancer; mTOR, mammalian target of rapamycin; NR, not reported/reached; ORR, overall response rate; OS, overall survival; PBO, placebo; PFS, progression‐free survival; PI3K, phosphoinositide‐3‐kinase; TTP, time to progression.