Table 1. Optimization of reaction conditions for enantioselective 3-exo-iodocyclization of allyl alcohol 1a a .
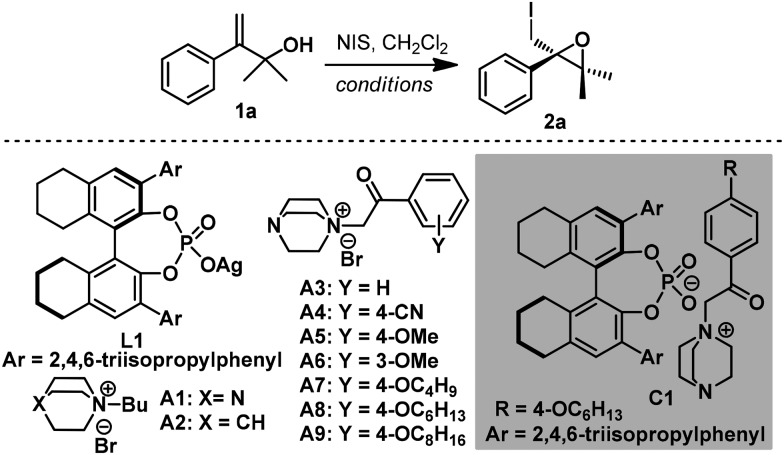
| ||||||
| Entry | Cat. (equiv.) | Additive (equiv.) | T (°C) | t (h) | Yield b (%) | ee c (%) |
| 1 | L1 (0.1) | A1 (0.12) | 0 | 40 | 16 | 30 |
| 2 | L1 (0.1) | A2 (0.12) | 0 | 40 | 18 | 19 |
| 3 | L1 (0.1) | A3 (0.12) | 0 | 40 | 44 | 77 |
| 4 | L1 (0.1) | A4 (0.12) | 0 | 40 | 16 | 69 |
| 5 | L1 (0.1) | A5 (0.12) | 0 | 40 | 69 | 86 |
| 6 | L1 (0.1) | A6 (0.12) | 0 | 40 | 47 | 80 |
| 7 | L1 (0.1) | A7 (0.12) | 0 | 40 | 65 | 91 |
| 8 | L1 (0.1) | A8 (0.12) | 0 | 40 | 60 | 92 |
| 9 | L1 (0.1) | A9 (0.12) | 0 | 40 | 50 | 91 |
| 10 | — | — | 0 | 40 | ND | — |
| 11 | L1 (0.1) | — | 0 | 40 | ND | — |
| 12 | — | A8 (0.12) | 0 | 40 | ND | — |
| 13 | C1 (0.1) | — | 0 | 40 | 42 | 83 |
| 14 | C1 (0.1) | A8 (0.1) | 0 | 40 | 82 | 92 |
| 15 | C1 (0.1) | S PPh3 (0.1) | 0 | 40 | 63 | 90 |
| 16 d | C1 (0.1) | A8 (0.1) | 0 | 40 | 62 | 69 |
| 17 e | C1 (0.1) | A8 (0.1) | 0 | 40 | 31 | 67 |
| 18 | C1 (0.1) | A8 (0.1) | –20 | 107 | 99 | 94 |
aCH2Cl2 (1 mL) was added to a mixture of silver salt L1 (0.01 mmol), ammonium salt A (0.012 mmol) and NIS (0.12 mmol), and the reaction mixture was cooled to 0 °C. Allyl alcohol 1a (0.1 mmol) in 0.5 mL CH2Cl2 was then added dropwise, and the reaction was quenched at the indicated time.
bIsolated yield.
cDetermined by HPLC using a Chiralpak AD column.
dCHCl3 as solvent.
eEtOAc as solvent. ND = not detected.
