Figure 1.
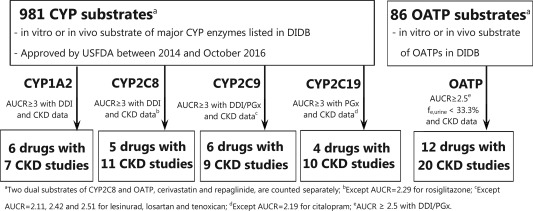
Overview of the workflow of clinical chronic kidney disease (CKD) data collection for cytochrome P450 (CYP)1A2, CYP2C8, CYP2C9, CYP2C19, and organic anion transporting polypeptide (OATP) model substrate drugs. AUCR, area under the concentration‐time curve ratio; DDI, drug‐drug interaction; DIDB, The University of Washington Metabolism and Transport Drug Interaction Database; fe,urine, fraction of the dose eliminated into urine unchanged; PGx, pharmacogenetics; USFDA, US Food and Drug Administration.
