Figure 1.
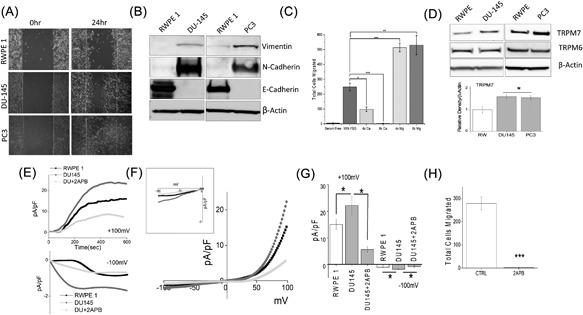
(A) Respective images showing the wound‐healing assay for cellular migration in human prostate epithelial cell RWPE1 and human prostate cancer cell line DU145 and PC3. Images were taken after the wound scratch (0 h) and after 24 h. The pictures are representative of 4 separate experiments performed in duplicate. (B) Western blots showing the expression of Vimentin, N‐cadherin, E‐cadherin, and loading control β‐actin in RWPE1, DU145, and PC3 cells. (C) DU145 cells were migrated using transwell inserts with altered Ca2+ and Mg2+ concentrations in both the upper and lower wells and cells were allowed to migrate for 6 h. 4× and 8× Ca2+ (1.6 mM and 3.2 mM) and Mg2+ (1.6 mM and 3.2 mM) concentrations were made by the addition of CaCl2 or MgCl2 to RPMI + 10% FBS. Four random fields at 10× were counted from three separate experiments indicating total cells migrated in each conditions ± SEM * indicate significance (*P < 0.05, ***P < 0.001). (D) Western blots showing the expression of TRPM6 and TRPM7 and loading control β‐actin in RWPE1, DU145, and PC3 cells. Corresponding densitometric reading of the TRPM7 protein is shown as a bar diagram and each bar is the mean ± SEM of three separate experiments. * indicates significance (P < 0.05). (E) In normal SES (1 mM Ca2+, 1 mM Mg2+) bath solution, whole cell recording showing outward/inward currents at +100 mV/‐100 mV in RWPE1 and DU145 cells. IV curves under various conditions as labelled are shown in (F) (Insert indicate the magnification of inward IV curves) and quantitation (8‐10 recordings) of current intensity at ±100 mV is shown in (G). * indicate significance (P < 0.05). (H) DU145 cells were placed in transwell inserts with RPMI medium with and without 50 μM 2‐APB. Nonmigrating cells were removed from upper membrane and migrated cells were fixed and stained with hematoxylin. Four random fields at 10× were counted from four separate experiments indicating total cells migrated ± SEM. *** indicates significance (P < 0.001)
