Figure 2.
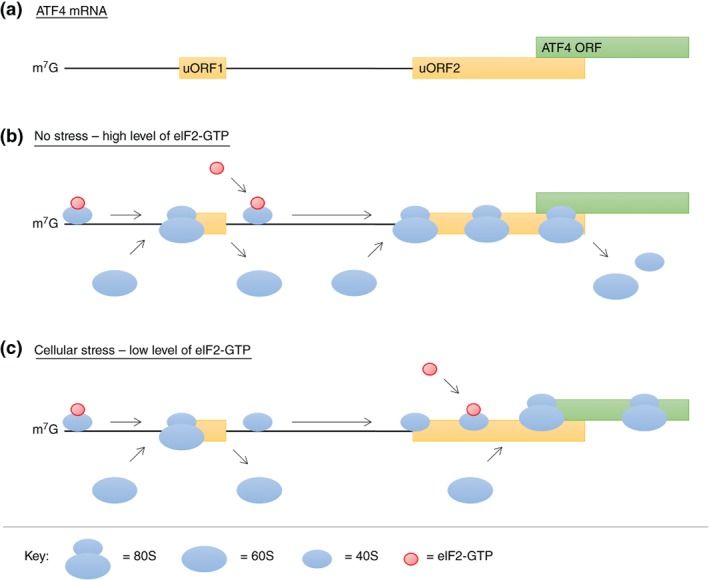
uORFs and cellular stress. ATF4 is a transcription factor regulated at the level of transcription and translation in response to stress (Dey et al., 2010), and plays an important role in the integrated stress response (ISR) by enhancing the expression of stress response transcripts (Harding et al., 2000; Vattem & Wek, 2004; Wek, Jiang, & Anthony, 2006). ATF4 mRNA translation is mediated by a delayed re‐initiation mechanism at two uORFs (Vattem & Wek, 2004). (a) ATF4 uORF1 is very short (encoding three amino acids) and functions in a positive manner, enhancing re‐initiation at downstream uORF2 (encoding 59 amino acids) which overlaps with the ATF4 ORF. (b) Under normal conditions, when eIF2‐GTP (and thus TC) availability is high, ribosomes translate uORF1 and the 60S ribosome dissociates. Importantly, the 40S ribosome remains associated and continues to scan the message, reinitiating at the inhibitory uORF2 and bypassing the ATF4 CDS (inhibiting ATF4 translation). (c) In response to cellular stress, when eIF2 is phosphorylated and eIF2‐GTP (and thus TC) availability is low, the scanning 40S ribosome bypasses uORF2 and instead re‐initiates at the start of the ATF4 ORF, increasing ATF4 translation. Therefore, delayed re‐initiation provides a mechanism for cells to selectively enhance the translation of mRNAs, particularly those encoding proteins required for adaptation and recovery from stress, during global protein synthesis inhibition
