Figure 1.
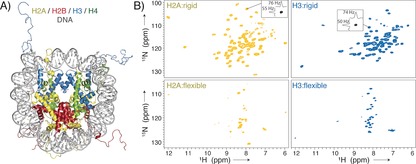
Nucleosome sedimentation yields high‐quality protein “fingerprint” NMR spectra covering both the globular core and flexible tails of the histones. A) The nucleosome is formed by an octamer of four core histones, H2A, H2B, H3, and H4, and binds ca. 147 bp of DNA around its surface (crystal structure from PDB ID 1KX515). B) 1H‐detected 2D NH spectra of sedimented nucleosomes with either 2H/15N/13C‐labeled H2A or H3, using either dipolar‐coupling‐based (top) or scalar‐coupling‐based (bottom) magnetization transfer. Slices along the 1H/15N dimension in the dipolar spectra are shown to highlight typical linewidths.
