Figure 2.
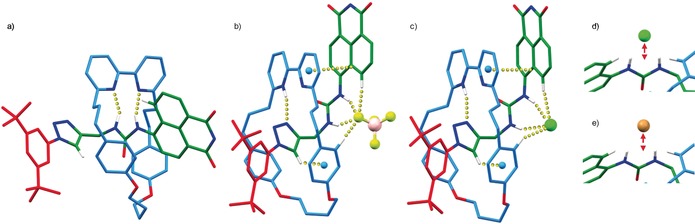
SCXRD structures with selected angles [°] and distances [Å] of a) 1 (NH1⋅⋅⋅N=2.45, NH2⋅⋅⋅N=2.32, C‐N1‐C‐Cipso=16.1), b) 1⋅HBF4 (NH1⋅⋅⋅F=1.99, NH2⋅⋅⋅F=2.16, CHG⋅⋅⋅F=2.56, CHh⋅⋅⋅F=2.16, H(py)⋅⋅⋅N=2.05, Hl⋅⋅⋅centroid=2.91, centroid⋅⋅⋅naphthalimide=3.39, C‐N1‐C‐Cipso=11.83), c) 1⋅HCl (NH1⋅⋅⋅Cl=2.24, NH2⋅⋅⋅Cl=2.42, CHG⋅⋅⋅Cl=2.92, CHh⋅⋅⋅Cl=2.59, H(py)⋅⋅⋅N=2.64, Hl⋅⋅⋅centroid=2.80, centroid⋅⋅⋅naphthalimide=3.42, C‐N1‐C‐Cipso=32.43). Anion binding unit showing the displacement of the anion from the H1‐H2‐HG‐Hh plane for d) 1⋅HCl (1.61 Å) and e) 1⋅HBr (1.84 Å). 1,1′‐Diphenylethyl substituents omitted for clarity.
