Abstract
Background
ADS‐5102 (amantadine) extended release capsules (GOCOVRI™) are a treatment for dyskinesia in patients with Parkinson's disease (PD). ADS‐5102 reduced dyskinesia and OFF time in phase 3 controlled trials of up to six months. Amantadine immediate release (IR) is used for dyskinesia, but suboptimal durability and tolerability limit its clinical utility.
Methods
In an ongoing, open‐label, phase 3 study in the US and Western Europe (NCT02202551), patients with PD received 274 mg of ADS‐5102 (equivalent to 340 mg amantadine HCl) once daily at bedtime for up to two years. Study outcomes included safety and assessment of motor complications, as measured by the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) Part IV. This manuscript focuses on those patients switched to ADS‐5102 from amantadine IR. Results in two groups of patients who previously completed a randomized controlled trial (EASE LID or EASE LID 3) are also presented according to use of ADS‐5102 or placebo in that study before enrollment in the open‐label study.
Results
Change in MDS‐UPDRS Part IV at week 8 was –0.3 in the previous ADS‐5102 subgroup (n = 61), –3.4 in the previous placebo subgroup (n = 79), and –3.4 in the previous amantadine IR subgroup (n = 32). Effects were maintained to week 64. In the previous amantadine IR subgroup (mean treatment duration, 2.5 years), mean amantadine IR dose was 221 mg. Safety data were consistent with previous randomized controlled trials of ADS‐5102.
Conclusion
These open‐label data suggest ADS‐5102 provides incremental reduction from baseline in MDS‐UDPRS Part IV score in patients switched directly from amantadine IR, without exacerbating adverse events.
Keywords: dyskinesia, amantadine, Parkinson's disease, levodopa‐induced (5 max)
Introduction
Parkinson's disease (PD) is a progressive synucleinopathy and the second most common neurodegenerative disease in the US.1 Cardinal motor symptoms of PD are bradykinesia, rigidity, tremor, and postural instability that reflect reduced striatal dopamine resulting from progressive degeneration of dopaminergic neurons in the substantia nigra.2, 3 The dopamine precursor levodopa exerts robust efficacy for the motor symptoms of PD.4 However, this effect is complicated by the emergence of motor complications (including motor fluctuations [OFF] and dyskinesia).5, 6
Motor complications are reported by approximately 40% to 60% of patients after five years of levodopa therapy and by nearly all patients (90%) after 10 years.5 These motor complications are associated, among others, with impaired activities of daily living, decreased health‐related quality of life, increased healthcare utilization, increased caregiver burden, and falls.7, 8 Strategies to improve OFF time include increasing the dosage of levodopa and/or adding adjunctive medications, including enzymatic inhibitors (monoamine oxidase B, catechol‐O‐methyltransferase) and dopamine agonists, but such strategies can be limited by the emergence of dyskinesia.9 Nonsurgical treatments for dyskinesia remain an unmet need. The main strategies for managing dyskinesia in patients with PD include fractionating the levodopa dose, lowering the overall dose of dopaminergic agents as a further treatment option, and lowering the levodopa dose while adding a longer‐acting dopamine agonist, all of which have the potential to worsen the patient's PD symptoms or increase dyskinesias.9
Amantadine is an N‐methyl‐d‐aspartate (NMDA) receptor antagonist that has uncompetitive binding, blocking only the activated, open‐channel NMDA receptor.10 Amantadine immediate release (IR) was originally approved for influenza, with an additional indication for parkinsonism in 1970.11, 12 Initially Schwab (1969)13 and Parkes (1970)14 found that while 200 mg daily of amantadine hydrochloride IR (equivalent to 161 mg amantadine) can be well tolerated, higher doses may be more efficacious yet associated with a greater incidence of central nervous system side effects, especially hallucinations and sleep disruptions. While the NMDA‐receptor antagonism of amantadine IR has been used to treat dyskinesia, its suboptimal durability and tolerability have limited its clinical utility.15, 16 Despite the same body of trial evidence, there are differing recommendations for amantadine across the guidelines of various countries.17, 18
ADS‐5102 (amantadine) extended release capsules (GOCOVRI™), a novel formulation of amantadine, were designed to deliver a distinct concentration‐time profile of amantadine that is not achievable with amantadine IR. With bedtime dosing of ADS‐5102, its initial slow rise in plasma concentration overnight achieves twice the plasma level (∼1500 ng/mL) of a standard dose of amantadine IR, 100 mg bid during the early morning and throughout the day.19 The once‐daily dosing regimen of ADS‐5102 may also provide enhanced convenience compared with multiple dosing of amantadine IR.
Recently, two phase 3, double‐blind, placebo‐controlled efficacy studies demonstrated that ADS‐5102 significantly reduced dyskinesia, with a secondary benefit of reduction in OFF time, when administered once daily at bedtime.20, 21, 22 In the current analysis, we assess the tolerability and efficacy of open‐label ADS‐5102 (amantadine) extended release capsules in the subgroup of patients who were receiving amantadine IR and then directly switched to ADS‐5102 at enrollment in the EASE LID 2 open‐label study. For comparison, results in two groups of patients who previously completed a randomized controlled trial (EASE LID or EASE LID 3), defined according to their use of ADS‐5102 or placebo prior to enrollment, are also presented.
Methods
Between July 28, 2014, and March 10, 2016, patients were screened and evaluated for eligibility in an open‐label trial of ADS‐5102 for the treatment of dyskinesia in patients with PD (EASE LID 2). An interim analysis was performed with a data cut‐off date of December 2, 2016 to evaluate the safety and tolerability of ADS‐5102. Each site's institutional review board, research ethics board, or independent ethics committee approved study protocol and patient consent forms. All patients provided written informed consent prior to any study procedures.
Patients were recruited after completing participation in one of the ADS‐5102 controlled, double‐blind trials (EASE LID20 or EASE LID 322), or the dose‐finding EASED study.21 Patients who were ineligible to enroll in an ADS‐5102 trial because they had undergone deep brain stimulation (DBS) were recruited to this open‐label trial. A full review of the methods and study results from this protocol have been previously published.23
This analysis is focused on the subgroup of patients in the EASE LID 2 open‐label study who were receiving amantadine IR at enrollment (confirmed through review of medication logs) and were switched at enrollment/baseline to ADS‐5102 (“previous amantadine IR subgroup”). Published study data from EASE LID 2 trial patients who received ADS‐5102 (“previous active”) or placebo (“previous placebo”) in the preceding randomized‐controlled studies and enrolled in the open‐label trial were included in this analysis to provide context (see Fig. 1). Previous data for DBS patients is not available as these patients were not eligible for previous ADS‐5102 phase 3 studies.
Figure 1.
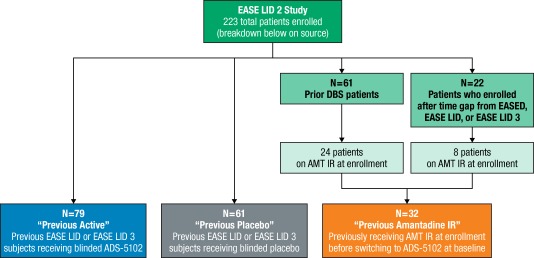
Enrollment and subgroup overview.
Abbreviations: AMT IR, amantadine immediate release; DBS, deep brain stimulation.
Statistical Analyses
All patients who received at least one dose of study drug during the open‐label study of ADS‐5102 were included in the safety analysis. All patients who received at least one dose of study drug and provided at least one post‐baseline Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) assessment were included in the efficacy analysis. Data were summarized by subgroup using descriptive statistics. Changes from baseline were analyzed using a mixed‐model repeated measure (MMRM) approach with categorical effects for subgroup (i.e., previous active, previous placebo, previous amantadine IR), week (8, 16, 28, 40, 52, and 64), interaction between subgroup and week, and an unstructured variance‐covariance matrix for repeated random‐subject effects. To control for known confounders to the changes in MDS‐UPDRS Part IV, baseline MDS‐UPDRS Part IV score, age at PD diagnosis, duration of levodopa treatment, duration of dyskinesia, and baseline levodopa dose were included as continuous covariates. Least squares mean (LSM) and P values derived from the statistical model are presented.
Results
Patients
In total, 32 patients were included in the “previous amantadine IR subgroup” at time of EASE LID 2 enrollment: 24 patients were post‐DBS (average duration 4–6 y) and eight patients previously completed the EASED study but had a gap between studies.21 Enrollment in EASE LID 2 had been completed when this analysis was performed. Treatment duration and study disposition are shown in Table 1 for all subgroups.
Table 1.
Treatment Duration and Study Disposition
|
Previous Active Group
a
(n = 61) |
Previous Placebo Group
a
(n = 79) |
Previous Amantadine IR
b
(n = 32) |
|
|---|---|---|---|
| Treatment Duration (days) | |||
| Mean (SD) | 419.9 (172.53) | 339.7 (193.38) | 406.8 (218.29) |
| Median | 389.0 | 340.0 | 430.0 |
| Min, Max | 56, 717 | 10, 713 | 9, 721 |
| Disposition | |||
| Ongoing | 46 (75.4) | 48 (60.8) | 17 (53.1) |
| Completed | 3 (4.9) | 3 (3.8) | 4 (12.5) |
| Discontinued | 12 (19.7) | 28 (35.4) | 11 (34.4) |
| Reasons for discontinuation | |||
| Adverse Event | 3 (4.9) | 16 (20.3) | 5 (15.6) |
| Consent withdrawn | 0 | 2 (2.5) | 0 |
| Unwilling to proceed | 0 | 4 (5.1) | 3 (9.4) |
| Lost to follow up | 0 | 1 (1.3) | 1 (3.1) |
| Needed to take exclusionary medication | 0 | 3 (3.8) | 1 (3.1) |
| eGFR less than 50 mL/min/m2 | 1 (1.6) | 0 | 0 |
| Other | 8 (13.1) | 2 (2.5) | 1 (3.1) |
| Patients with at least 28 weeks of treatment | 57 (93.4) | 59 (74.7) | 26 (81.3) |
| Patients with at least 52 weeks of treatment | 32 (52.5) | 37 (46.8) | 20 (62.5) |
| Patients with at least 76 weeks of treatment c | 18 (29.5) | 14 (17.7) | 8 (25.0) |
aThese patients were previously enrolled in either EASE LID or EASE LID 3.
bPatients with prior DBS (n = 24) or who participated in EASED study (n = 8).
cStudy is ongoing.
Abbreviations: DBS, deep brain stimulation; eGFR, estimated glomerular filtration rate; IR, immediate release; SD, standard deviation.
At baseline, the mean reported duration of amantadine IR treatment was 2.5 years (0.1–14.4 y) and the mean amantadine IR dose was 221 mg/day, equivalent to 275 mg of amantadine HCl.
Baseline demographics and PD characteristics are included in Table 2. On average, previous amantadine IR patients were approximately five years younger than patients enrolled directly from the double‐blind studies (“previous active” and “previous placebo” subgroups; mean age 60.6 y) and predominantly male (68.8%). Mean duration of dyskinesia in previous amantadine IR patients was 7.4 years, compared with 4.6 and 4.0 years in the previous active and previous placebo subgroups, respectively. Baseline MDS‐UPDRS Part IV scores for previous amantadine IR subgroup were similar to scores of the previous placebo subgroup (∼9.5–10).20, 22
Table 2.
Baseline Demographics and PD Characteristics
|
Previous Active Group
a
(n = 61) |
Previous Placebo Group
a
(n = 79) |
Previous Amantadine IR b (n = 32) |
|
|---|---|---|---|
| Age (years), Mean (SD) | 64.2 (9.9) | 65.8 (8.8) | 60.6 (11.1) |
| Male, n (%) | 34 (55.7) | 45 (57.0) | 22 (68.8) |
| Years since DBS, Mean (SD) | n/a | n/a | 5.8 (4.1) c |
| Age at PD diagnosis (years), Mean (SD) | 53.4 (10.1) | 56.1 (8.4) | 46.7 (11.4) |
| Years since PD diagnosis, Mean (SD) | 11.2 (4.5) | 10.0 (4.1) | 14.5 (7.6) |
| Duration of levodopa treatment (years), Mean (SD) | 8.7 (3.7) | 7.8 (4.0) | 10.9 (6.1) |
| Baseline levodopa dose, mg/day, Mean (SD) | 805.0 (421.4) | 754.3 (556.2) | 590.3 (359.8) |
| Duration of dyskinesia (years), Mean (SD) | 4.6 (3.4) | 4.0 (2.5) | 7.4 (4.2) |
| Years of prior amantadine exposure, Mean (SD) | n/a | n/a | 2.5 (2.9) |
| Total daily amantadine IR HCl dose (mg), Mean (SD) | n/a | n/a | 275 (81.5) d |
| MDS‐UPDRS Part III, Mean (SD) (in ON state) | 21.4 (11.3) | 21.9 (13.1) | 28.1 (13.4) |
| MDS‐UPDRS Part IV Total, Mean (SD) | 6.5 (3.4) | 9.6 (3.1) | 9.8 (3.2) |
| Item 4.1, Mean (SD) | 1.6 (1.1) | 1.9 (1.1) | 1.9 (0.9) |
| Item 4.2, Mean (SD) | 0.9 (1.0) | 1.8 (0.9) | 2.2 (0.9) |
| Item 4.3, Mean (SD) | 0.9 (0.6) | 1.3 (0.6) | 1.2 (0.6) |
| Item 4.4, Mean (SD) | 1.4 (1.2) | 1.9 (1.3) | 2.1 (1.2) |
| Item 4.5, Mean (SD) | 1.2 (1.1) | 1.5 (1.1) | 1.4 (1.0) |
| Item 4.6, Mean (SD) | 0.6 (1.0) | 0.9 (1.1) | 1.0 (1.3) |
Data from patients from the phase 3 study who enrolled into the open‐label, previously published in Hauser et al.,23 are provided for context.
aThese patients were previously enrolled in either EASE LID or EASE LID 3.
bPatients with prior DBS (n = 24) or who participated in EASED study (n = 8).
cData for patients with prior DBS only (n = 24).
dDose 275 (81.5) mg is for amantadine HCl; amantadine dose is 221 mg (range: 100–400 mg/day amantadine HCl, equivalent to 81–338 mg/day amantadine).
Abbreviations: DBS, deep brain stimulation; IR, immediate release; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale; PD, Parkinson's disease; SD, standard deviation.
MDS‐UPDRS
Fig. 2A shows the mean MDS‐UPDRS Part IV scores over time for all three cohorts. Similar to patients in the previous placebo subgroup, MDS‐UPDRS Part IV scores in the previous amantadine IR subgroup decreased from baseline to the first post‐baseline visit (week 8) and were sustained to week 64. At week 8, the LSM treatment difference between the previous amantadine IR subgroup and previous active subgroup was –2.8 units (P = 0.046), and the LSM treatment difference between the previous placebo and previous active subgroups was –2.5 units (P = 0.055; Supporting Table 1). The mean percent change from baseline in MDS‐UPDRS Part IV at week 8 for all subgroups is shown in Fig. 2B.
Figure 2.
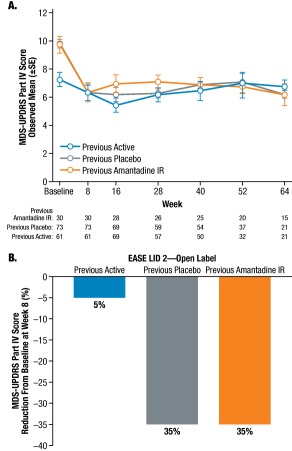
MDS‐UPDRS part IV (motor complications) over time. (A) Observed changes from baseline in three patient subgroups; (B) Mean percentage score reductions from baseline at first post‐baseline visit (Week 8) in patient subgroups.
Abbreviations: IR, immediate release; MDS‐UPDRS, Movement Disorder Society‐Unified Parkinson's Disease Rating Scale; SE, standard error.
To identify which subscales drove the reduction in Part IV, the mean change from baseline to week 8 were analyzed for each subgroup and the results are shown in Fig. 3. The MDS‐UPDRS Part IV change from baseline to week 8 was –0.3 in the previous active subgroup (n = 61), –3.4 in the previous placebo subgroup (n = 79), and –3.4 in the previous amantadine IR subgroup (n = 32). Both the previous placebo and the previous amantadine IR subgroups showed comparable changes in MDS‐UPDRS Part IV subscale Items 4.1 to 4.4. Fig. 4A and Fig. 4B show the distribution of change across MDS‐UPDRS Part IV overall and across its subscales at week 8.
Figure 3.
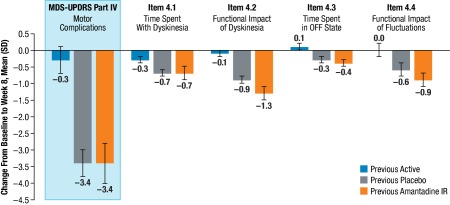
Change from baseline to week 8 in MDS‐UPDRS Part IV total score and changes in Part IV subscales across patient subgroups.
No effect was observed in MDS‐UPDRS Part IV subscale items 4.5 and 4.6 in any patient subgroup.
Abbreviations: MDS‐UPDRS, Movement Disorder Society‐Unified Parkinson's Disease Rating Scale; SD, standard deviation.
Figure 4.
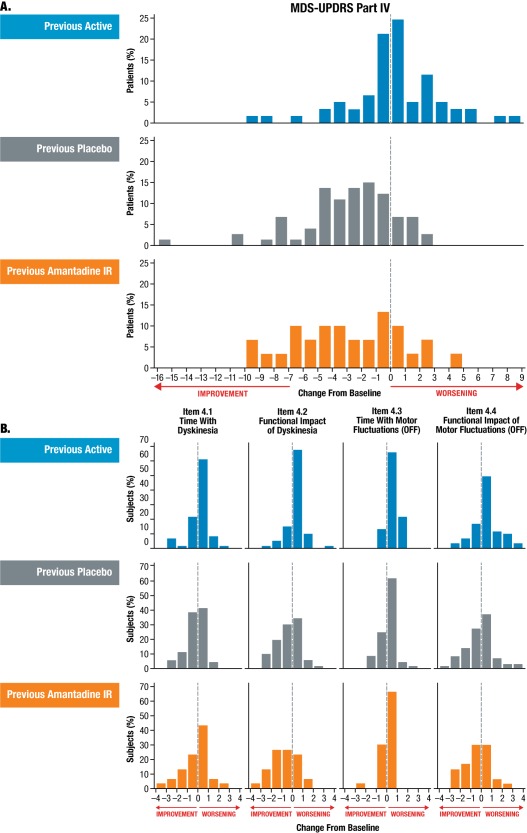
Distribution of change in MDS‐UPDRS, Part IV (A) and subscales (B) across patient subgroups at week 8.
No effect was observed in MDS‐UPDRS Part IV subscale items 4.5 and 4.6 in any patient subgroup.
Abbreviations: IR, immediate release; MDS‐UPDRS, Movement Disorder Society‐Unified Parkinson's Disease Rating Scale.
Additionally, at the time of the data cut, approximately 25% of patients in each subgroup were able to increase their levodopa dose while on the study (median increase from 225 mg to 325 mg; Supporting Table 2).
Adverse Events
An overview of adverse events (AEs) is provided in Table 3. In the total study population (N = 223), falls, visual hallucination, peripheral edema, and constipation were each reported by ≥ 10% of patients; AEs were the primary reason for study‐drug discontinuation in 32 patients (14.3%). The most common AEs leading to discontinuation (reported by ≥ 2% patients) within the previous amantadine IR subgroup were falls (6.3%, n = 2). In the overall open‐label study population, the most common AEs leading to discontinuation were falls (2.2%, n = 5), visual hallucinations (2.2%, n = 5), and peripheral edema (0.9%, n = 2).23
Table 3.
Overview of Adverse Events (AEs) Data from patients from the phase 3 study who enrolled in the open‐label, previously published in Hauser et al.,23 are provided for context.
|
Previous Active Group
a
(n = 61) |
Previous Placebo Group
a
(n = 79) |
Previous Amantadine IR
b
(n = 32) |
|
|---|---|---|---|
| No. (%) of patients with any | |||
| AE | 53 (86.9) | 69 (87.3) | 27 (84.4) |
| Study drug–related AE | 29 (47.5) | 41 (51.9) | 14 (43.8) |
| Serious AE (SAE) | 11 (18.0) | 13 (16.5) | 5 (15.6) |
| Study drug–related SAE | 0 | 1 (1.3) | 1 (3.1) |
| No. (%) of patients who permanently discontinued treatment due to any | |||
| AE | 5 (8.2) | 16 (20.3) | 5 (15.6) |
| Study drug–related AE | 1 (1.6) | 12 (15.2) | 5 (15. 6) |
| Most common AEs (≥5% all patients), n (%) | |||
| Fall | 12 (19.7) | 20 (25.3) | 8 (25.0) |
| Visual hallucination | 10 (16.4) | 20 (25.3) | 6 (18.8) |
| Peripheral edema | 9 (14.8) | 9 (11.4) | 7 (21.9) |
| Dizziness | 0 | 8 (10.1) | 5 (15.6) |
| Constipation | 9 (14.8) | 9 (11.4) | 2 (6.3) |
| Livedo reticularis | 6 (9.8) | 4 (5.1) | 3 (9.4) |
| Nausea | 7 (11.5) | 6 (7.6) | 1 (3.1) |
| Dry mouth | 3 (4.9) | 6 (7.6) | 1 (3.1) |
aThese patients were previously enrolled in either EASE LID or EASE LID 3.
bPatients with prior DBS (n = 24) or who participated in EASED study (n = 8).
Note: A patient was counted once within each preferred term. Percentages were calculated based on the number of patients in each group.
Abbreviations: IR, immediate release.
The incidence of serious AEs (SAEs) was ∼17% and comparable among the three cohorts; all but two SAEs (urinary tract infection [previous placebo] and suicidal ideation [previous amantadine IR]) were considered not related to study drug. Falls, visual hallucinations, peripheral edema, and dizziness were the most commonly reported AEs in the previous amantadine IR subgroup, consistent with the other subgroups. In general, vital signs and laboratory results remained consistent with baseline values and were similar across all subgroups. In the previous amantadine IR subgroup, there were no reports of orthostatic hypotension or impulse‐control disorder.
Discussion
Patients receiving amantadine IR for an average of 2.5 years entered the EASE LID 2 open‐label study with motor complication scores similar to those of patients previously treated with placebo in the controlled trials. Of the 32 patients receiving amantadine IR who were switched at enrollment, 20 completed at least one year of treatment, 26 completed at least six months, and 17 remain ongoing. After switching from amantadine IR directly to ADS‐5102 at bedtime (137 mg for one week, 274 mg thereafter), these patients experienced an improvement in motor complications, as shown by a reduction in their MDS‐UPDRS Part IV scores at the first post‐baseline visit (week 8). Additionally, more patients were able to increase rather than decrease their levodopa dose (total mg) without compromising dyskinesia control, which would not be expected in a cohort of patients with dyskinesia >4 years' duration.9 This treatment effect was also equivalent to that shown in the previous placebo subgroup and was maintained for over a year. The reduction in MDS‐UPDRS Part IV scores was driven both by decreases in overall time and functional impact of dyskinesia and OFF time.
The similarity in baseline MDS‐UPDRS Part IV scores between the Previous Placebo and Previous Amantadine IR group is noteworthy. These patients were enrolled under inclusion criteria that stated that patients could be enrolled who experienced peak dose dyskinesia during screening that might benefit from specific dyskinesia treatment in the judgment of the subject and clinical investigator, or they had a history of peak dose dyskinesia that was currently being managed by amantadine treatment. So, although the reason they were experiencing dyskinesia or motor complications is not known, they were experiencing refractory dyskinesia at entry, despite being on an average of 275 mg amantadine HCl, and most having had DBS.
The safety profile in patients who switched from amantadine IR directly to ADS‐5102 was consistent with previously reported safety data from the controlled and open‐label clinical trials.23
The primary limitations of these analyses are the nonrandomized, open‐label design, and the small sample size of the previous amantadine IR subgroup. The absence of MDS‐UPDRS, Part III during the OFF state is another limitation. Also, most of the patients in the previous amantadine IR subgroup were status‐post DBS implantation (n = 24; 75%), were younger, and had a longer history of dyskinesia. The MMRM analysis was adjusted for several clinically important baseline covariates. However, the presence of additional unknown and potentially biasing factors cannot be discounted.
The results seen for the two comparison cohorts (i.e., the small response seen in the previous active subgroup and the improvement in the previous placebo subgroup) are consistent with recent findings from the double‐blind studies and also support the internal and external validity of the evaluation of the previous amantadine IR group. This indicates that the treatment effect seen in the previous amantadine IR subgroup was not only due to an open‐label trial effect.
These open‐label data are encouraging and support the premise that higher sustained daytime concentrations of amantadine provided by ADS‐5102 (∼1500 ng/mL) provide a further reduction in dyskinesia and OFF time in patients previously treated with amantadine IR. As seen in the other subgroups, this effect was sustained up to 64 weeks. ADS‐5102 also provided benefits in dyskinesia and OFF in a population already receiving amantadine IR treatment and DBS.
These data suggest that patients currently being treated with amantadine IR can be switched directly to ADS‐5102 without interruption. Patients' ability to switch from amantadine IR bid or tid to a once‐daily regimen of ADS‐5102 may provide dosing convenience benefits and supports the premise that higher sustained daytime concentrations of amantadine provided by ADS‐5102 (approx.1500 ng/mL) provide a further reduction in dyskinesia and OFF in previously treated amantadine IR patients.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
S.H.I.: 1A, 1B, 1C, 2C, 3A, 3B, 3C
S.F.: 1C, 2C, 3B
RPah: 1A, 1B, 1C, 2C, 3B
C.M.T.: 1C, 2C, 3B
A.J.E.: 1C, 2C, 3B
C.T.: 1A, 1B, 1C, 2C, 3B
C.H.A.: 1C, 2C, 3B
RPat: 1A, 1B, 2A, 2C, 3B
R.J.: 1A, 1B, 2A, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This study was funded by Adamas Pharmaceuticals, Inc.
Financial Disclosures for the previous 12 months: Stuart H. Isaacson has received honoraria for CME, consultant, research grants, and/or promotional speaker on behalf of AbbVie, Acadia, Acorda, Adamas Pharmaceuticals, Addex, Allergan, Amarantus, Auspex, Avid, Axovant, AZ Therapies, Biogen, Biotie, Britannia, Cynapsus, Eisai, Eli Lilly, GE Healthcare, Impax, Intec Pharma, Ipsen, Kyowa, Lundbeck, Medtronics, Merz, Michael J. Fox Foundation, Neurocrine, Neuroderm, NINDS/NIH, Parkinson Study Group, Pfizer, Pharma2B, Prothena, Roche, Sanofi, Shire, Sunovion, Teva, UCB, US WorldMeds, and XenoPort. Stanley Fahn has received consulting fees from Teva Pharmaceuticals, Intra‐Cellular Therapies, Inc, Adamas Pharmaceuticals, Retrophin Inc, PhotoPharmics, Kirkland & Ellis, Sun Pharma Advanced Research Co. Ltd, and Kashiv Pharma, and received grant/research support from Smart Family Foundation. He has received personal fees from Springer Publishers for serving as co‐editor of Current Neurology and Neurosurgery Report (annual) and receives royalties from Elsevier Publishers for co‐authorship of book Principles and Practice of Movement Disorders. Rajesh Pahwa is receiving or has received honoraria or payments for consulting from AbbVie, Acadia, Acorda, Adamas Pharmaceuticals, Cynapsus, Impax, Lundbeck, Medtronic, Neurocrine, Sage, St Jude Medical, Teva Neuroscience, Medtronic, UCB, and US WorldMeds. He has received research grants from Acadia, Acorda, Adamas Pharmaceuticals, Avid, Biotie, Boston Scientific, Civitas, Cynapsus, Kyowa, NIH/NINDS, NPF, Pfizer, and PSG/University of Rochester. He has also served on the data monitoring committee for Ceregene. He has received personal compensation as the Co‐Editor‐in‐Chief of the International Journal of Neuroscience. Caroline M. Tanner is an employee of the University of California‐San Francisco and the San Francisco Veterans Affairs Medical Center and an intermittent employee of the Parkinson's Institute. She serves on the Scientific Advisory Boards of the Michael J. Fox Foundation and the National Spasmodic Dysphonia Association as a voluntary consultant and has provided paid consulting services to Ultragenyx Pharmaceuticals, Neurocrine Biosciences, Cynapsus Therapeutics, Sage Biometrics, and Adamas Pharmaceuticals. She has received compensation for serving on Data Monitoring Committees from Biotie Therapeutics, Voyager Therapeutics, and Intec Pharma. She receives grant support from the Michael J. Fox Foundation, the Parkinson's Disease Foundation, the Department of Defense, and the National Institutes of Health. Alberto J. Espay has received grant support from the National Institutes of Health, Great Lakes Neurotechnologies, and the Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for AbbVie, Teva, Impax, Acadia, Cynapsus, Lundbeck, and US WorldMeds; personal compensation as a member of the speaker bureau for AbbVie, Impax, Acadia, Lundbeck, and US WorldMeds; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. Claudia Trenkwalder reports personal fees from Mundipharma Research GmbH & Co; payment for advisory board from Mundipharma Germany GmbH & Co., UCB, Vifor, Britannia, Novartis; payment for lectures from UCB, Desitin, Britannia. Charles H. Adler has received consulting fees from Acadia, Acorda, Adamas, Extera Partners, Jazz, Lundbeck, Minerva, Neurocrine, Revance, Scion, and Sunovion, and received research funding from the Michael J. Fox Foundation for Parkinson's Research, and the NIH/NINDS. Rajiv Patni and Reed Johnson are employees and own stock in Adamas Pharmaceuticals.
Supporting information
Table S1. Efficacy results for MDS‐UPDRS Part IV (efficacy population).
Table S2. Levodopa dose changes from baseline.
Funding informaton: This study was funded by Adamas Pharmaceuticals, Inc.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003;157(11):1015–1022. [DOI] [PubMed] [Google Scholar]
- 2. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311(16):1670–1683. [DOI] [PubMed] [Google Scholar]
- 3. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013;136(Pt 8):2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS‐ES review on therapeutic management of Parkinson's disease. Eur J Neurol 2013;20(1):5–15. [DOI] [PubMed] [Google Scholar]
- 5. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16(3):448–458. [DOI] [PubMed] [Google Scholar]
- 6. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009;72(21 Suppl 4):S1–136. [DOI] [PubMed] [Google Scholar]
- 7. Suh DC, Pahwa R, Mallya U. Treatment patterns and associated costs with Parkinson's disease levodopa induced dyskinesia. J Neurol Sci 2012;319(1–2):24–31. [DOI] [PubMed] [Google Scholar]
- 8. Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson's disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord 2014;20(9):969–974. [DOI] [PubMed] [Google Scholar]
- 9. Muller T, Woitalla D, Russ H, Hock K, Haeger DA. Prevalence and treatment strategies of dyskinesia in patients with Parkinson's disease. J Neural Transm (Vienna) 2007;114(8):1023–1026. [DOI] [PubMed] [Google Scholar]
- 10. Parsons CG, Quack G, Bresink I, et al. Comparison of the potency, kinetics and voltage‐dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 1995;34(10):1239–1258. [DOI] [PubMed] [Google Scholar]
- 11. Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology 2012;78(14):1096–1099. [DOI] [PubMed] [Google Scholar]
- 12. Symmetrel (amantadine hydrochloride, USP) [prescribing information]. Chadds Ford, PA: Endo Pharmaceuticals Inc; January 2009. [Google Scholar]
- 13. Schwab RS, England AC, Jr. , Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson's disease. JAMA 1969;208(7):1168–1170. [PubMed] [Google Scholar]
- 14. Parkes JD, Zilkha KJ, Marsden P, Baxter RC, Knill‐Jones RP. Amantadine dosage in treatment of Parkinson's disease. Lancet (London, England) 1970;1(7657):1130–1133. [DOI] [PubMed] [Google Scholar]
- 15. Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry 2004;75(1):141–143. [PMC free article] [PubMed] [Google Scholar]
- 16. Hayden FG, Gwaltney JM, Jr. , Van de Castle RL, Adams KF, Giordani B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrobial agents and chemotherapy 1981;19(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence‐Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S2–41. [DOI] [PubMed] [Google Scholar]
- 18. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66(7):983–995. [DOI] [PubMed] [Google Scholar]
- 19. Hauser RA, Pahwa R, Wargin W, Souza‐Prien C, McClure N, Patni R, Went GT. Pharmacokinetics of ADS‐5102 [amantadine] extended release capsules administered once‐daily at bedtime for the treatment of dyskinesia. [Submitted]. [DOI] [PMC free article] [PubMed]
- 20. Pahwa R, Tanner CM, Hauser RA, et al. ADS‐5102 (Amantadine) Extended‐Release Capsules for Levodopa‐Induced Dyskinesia in Parkinson Disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol 2017;74(8):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pahwa R, Tanner CM, Hauser RA, et al. Amantadine extended release for levodopa‐induced dyskinesia in Parkinson's disease (EASED Study). Mov Disord 2015;30(6):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo‐controlled trial of ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson's disease (EASE LID 3). Mov Disord 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hauser RA, Pahwa R, Tanner CM, et al. ADS‐5102 (Amantadine) Extended‐Release Capsules for Levodopa‐Induced Dyskinesia in Parkinson's Disease (EASE LID 2 Study): Interim Results of an Open‐Label Safety Study. J Parkinsons Dis 2017;7(3):511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Efficacy results for MDS‐UPDRS Part IV (efficacy population).
Table S2. Levodopa dose changes from baseline.


