Figure 2.
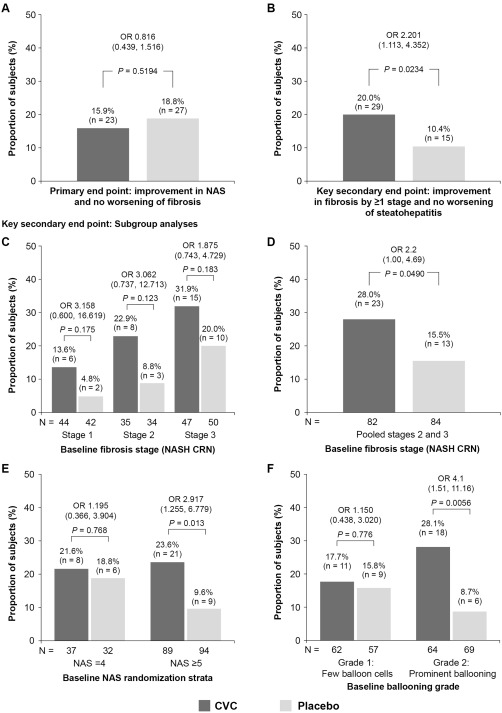
Primary endpoint and key secondary endpoint of improvement in fibrosis by ≥1 stage and no worsening of SH at year 1 (ITT analysis), with subgroup analyses for the key secondary endpoint (mITT population). (A) Subjects meeting the primary endpoint (improvement in NAS and no worsening of fibrosis). (B) Subjects meeting the key secondary endpoint of improvement in fibrosis by ≥1 stage and no worsening of SH. Missing biopsies were counted as treatment failure. (C,D,E,F) Response for the key secondary endpoint by baseline: (C) fibrosis stage (NASH CRN system); (D) fibrosis stages 2 and 3 pooled (NASH CRN system); (E) NAS stratification; and (F) hepatocellular ballooning grade. OR are presented with 95% CI and P values and were calculated using a logistic regression model with factors for randomized treatment group, NAS at screening (4 or ≥5), and fibrosis stage (≤2 or >2). Abbreviation: mITT, modified intent‐to‐treat.
