Figure 1.
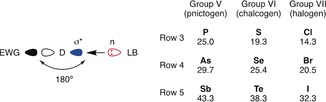
Pnictogen, chalcogen, and halogen bonds between the σ* orbital of a donor (D, with electron‐withdrawing groups (EWGs) to deepen the σ hole) and a lone pair n of a bound Lewis base (LB) afford bond angles around 180° (left) and are predicted to increase in strength with the polarizability of the donor in main groups V–VII (right; computational values based on MP2 calculations in a.u.).13
