Abstract
Insulin resistance in mice typically does not manifest as diabetes due to multiple compensatory mechanisms. Here we present a novel digenic model of type 2 diabetes in mice heterozygous for a null allele of the Insulin Receptor and an N-ethyl-N- nitrosourea induced alternative splice mutation in the regulatory protein phosphatase 2A (PP2A) subunit PPP2R2A. Inheritance of either allele independently results in insulin resistance but not overt diabetes. Doubly heterozygous mice exhibit progressive hyperglycaemia, hyperinsulinaemia and impaired glucose tolerance from 12 weeks of age without significant increase in bodyweight. Alternative splicing of Ppp2r2a decreased PPP2R2A protein levels. This reduction in PPP2R2A containing PP2A phosphatase holoenzyme was associated with decreased serine/threonine protein kinase AKT protein levels. Ultimately, reduced insulin stimulated phosphorylated AKT levels were observed, a result that was confirmed in Hepa1-6, C2C12 and differentiated 3T3-L1 cells knocked down using Ppp2r2a siRNAs. Altered AKT signaling and expression of gluconeogenic genes in the fed state contributed to an insulin resistance and hyperglycaemia phenotype. This model demonstrates how genetic changes with individually small phenotypic effects can interact to cause diabetes and how expression difference hypomorphic alleles of PPP2R2A and potentially other regulatory proteins have deleterious effects and may therefore be relevant in determining diabetes risk.
Type 2 diabetes is a complex disease where cellular resistance to insulin combined with a failure in beta cell compensation results in the development of disease. Underlying this process are multiple genetic and environmental factors that interact to determine susceptibility risk. However, there are relatively few examples of diabetes patients whose disease can be demonstrated to be due to the interaction of mutations in two of more genes. One of these is due to heterozygous mutations in 2 unlinked genes, peroxisome proliferator receptor gamma (PPARG) and protein phosphatase 1, regulatory (inhibitor) subunit 3A (PPP1R3A), expressed in adipocytes and skeletal muscle respectively and results in severe insulin resistance and lipodystrophy (1). A second example is haploinsufficiency for the insulin receptor (IR) in combination with chimerin-2 a GTPase-activating protein (CHN2) that results in insulin resistance and deficiency in interuterine growth (2). In this latter example the CHN2 mutation implicates a novel gene in insulin signaling and its regulation of metabolism and growth (2). Although there are other examples of doubly heterozygous individuals with diabetes, for example in the MODY HNF1A and HNF4A genes it is unclear how these impact the severity of disease (3). In a mouse model, a digenic insulin resistance phenotype has been described whereby 40% of mice heterozygous for both insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) null alleles develop overt diabetes at 4-6 months of age demonstrating how two mild impairments in the same pathway can interact to cause diabetes (4). The insulin-signaling pathway including IR, IRS, PI3K, AKT and its effectors, as well as pathways via ERK, regulate key metabolic processes including gluconeogenesis, glucose uptake, glycogen synthesis, lipogenesis, protein synthesis and growth (5–7). These highly regulated multistep pathways may be perturbed with multiple small effect mutations that collectively result in significant disruption and consequent disease (5).
Here we describe a digenic mouse model of type 2 diabetes where haploinsufficiency of insulin receptor and an N-ethyl-N-nitrosourea (ENU)-induced novel splice-site mutation in the protein phosphatase 2 PP2, regulatory subunit B, alpha gene (Ppp2r2a) gives rise to a diabetic phenotype as a result of aberrant AKT signaling. We demonstrate the synergistic effect of two mutations affecting insulin signaling leading to impaired glucose homeostasis when combined, supporting the concept that genetic susceptibility to diabetes can be determined by the interaction of small effect alleles.
Research Design and Methods
Animal Husbandry
Mice were kept in accordance with UK Home Office welfare guidelines and project licence restrictions, in addition the study was approved by the local Animal Welfare and Ethical Review Body. Insulin Receptor C57BL/6J knockout mice (8) were obtained from the Jackson Laboratories.
SNP mapping and next generation sequencing
Genomic DNA was extracted from mouse tail or ear biopsy tissue using a Qiagen DNeasy tissue Kit and 250ng was assayed against the Illumina Mouse Medium Density Linkage Panel (Illumina). F1 founder genomic DNA 4μg was fragmented by nebulization. The DNA-Seq library was further prepared from the fragmented DNA following the commercial instruction of sample preparation for sequencing genomic DNA (Illumina). Next-generation sequencing analysis was performed on ArraySuite® software OmicSoft®). After DNA-Seq alignment using Omicsoft Aligner to mouse mm10, mutations were identified using Omicsoft’s summarize mutation function. The mutation report was further annotated with Ensembl gene models and dbSNP database. A list of interesting ENU mutation candidates was obtained by focusing on newly discovered missense mutations and mutations which could affect splicing events in ENU regions.
PCR primers for amplifying ENU-induced candidate hits in Ppp2r2a and Imt2b from genomic DNA were based on the sequences in GenBank. Primer sequences for Ppp2r2a were 5’-CAGTCCCTGTCTGTCTGTAACATACTCAG-3’ and 5’- CCCTTCCCACCAGATCACTCTTTGTC-3’and for Itm2b were 5’- GCAAATTATCATATCTCTTTTGTCCGGATGCAC-3’ and 5’- GAATGTATATTTGAAGCTGGGCATGGCTG-3’. PCR-amplified gDNA or cDNA fragments were subcloned into the pCR II vector using a TA cloning kit (Life Technologies) and sequenced with M13F and M13R primers or directly sequenced with the PCR primers on an ABI 3730xl DNA Analyzer.
Mouse Phenotyping assays
Mice were tested using the EMPReSS simplified IPGTT (http://empress.har.mrc.ac.uk). Plasma glucose was measured using an Analox Glucose Analyser GM9. For nsulin tolerance tests (ITT), mice were fasted for 4 hours and a baseline blood sample taken followed by an intraperitoneal injection of 2 iU/kg of insulin, blood samples were then taken at 10, 20, 40 and 60 minutes and blood glucose determined using alphatrak glucometer. Plasma insulin was measured using a Mercodia Mouse Insulin ELISA kit. Mice were weighed at 2 week intervals between 12 and 30 weeks of age and placed in metabolic cages (Tecniplast) for 24 hour periods to measure food and water intake and urine output.
Insulin stimulation
Mice were fasted overnight, given a surgical anesthetic dose (isoflourane) and 5iU of insulin or saline injected directly into the hepatic portal vein, euthanized 90 seconds later and liver, gonadal fat pads and gastrocnemius muscles were excised and immediately frozen in liquid nitrogen.
Cell culture and siRNA knockdown
Hepa1-6, 3T3-L1 and C2C12 cells were purchased from ATCC and were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen) supplemented with either 10% fetal bovine serum (Invitrogen) or 10% calf serum (3T3-L1), 100units.ml-1 penicillin, and 100mg.ml-1 streptomycin (Invitrogen). Adipogenic differentiation of 3T3-L1 cells was induced by incubating cells in serum free media for 48 hours priorto supplementing the tissue culture medium with 250μM IBMX, 0.1μM dexamethasone and 0.5μg/ml insulin for 4 days. After this period, tissue culture medium was supplemented with insulin only.
Four siRNAs specific for Ppp2r2a were purchased from Qiagen, of which 2 oligos: 5’-TCCACGGAGAATATTTGCCAA-3’ (siRNA1) and 5’-AAGCATCACGAGAGAACAATA-3’ (siRNA3) gave greater than 70% knockdown, Stealth™ low-GC negative control was purchased from Invitrogen. siRNA was transfected into Hepa1-6 or C2C12 cells at 60-70% confluency at a final concentration of 30nM in 6-well plates, using Lipofectamine RNAiMax (Invitrogen). 3T3-L1 transfections were performed on Day 8 of differentiation. 30 hours after transfection cells were serum starved for 18 hours prior to incubation in serum free media supplemented with 500nM insulin or saline for 15 minutes. Cell lysates were collected for either RNA or protein extraction.
Glucose production assays
30 hours post transfection with siRNAs Hepa1-6 cells were incubated overnight in Glucose free DMEM media (0.1% BSA, 1mM Sodium Pyruvate). Cells were washed 3 times in PBS and incubated in Glucose production Buffer for 6 hours (Glucose free DMEM without phenol red, 20mM Sodium Lactate, 2mM Sodium Pyruvate, 2mM L-Glutamate, 15mM HEPES, 0.1% BSA) with or without 500nM insulin. Supernatant was collected and assayed for glucose concentration (Analox Glucose Analyser GM9), cells lysed and protein concentration quantified using a DC protein assay (Biorad). Secreted glucose concentration was normalized to total protein concentration per well.
Protein extraction and Simple Western blotting
Protein was extracted from frozen tissue by homogenization in Cell Lytic protein lysis buffer (Sigma) supplemented with protease and phosphatase inhibitor cocktails (Roche). Protein was quantified using a DC protein Assay (Biorad). Lysates of 1.3mg/ml protein (3.75μl) were mixed with 1.25μl of Simple Western™ sample dilution buffer (ProteinSimple) containing reducing agent and fluorescent standards to a final concentration of 1mg/ml, and denatured at 95ºC for 5 minutes, before analysis using automated capillary electrophoresis system PEGGY. Primary antibodies against PPP2R2A(5689), AKT(9272), GSK-3 (27C10), p70S6K(49D7) (Cell Signalling) and Tubulin (12G10 Developmental Studies Hybridoma Bank) were used in this study. Briefly, proteins were separated on the PEGGY instrument through size resolving matrix in capillaries, immobilized to the inner capillary wall, incubated with primary and secondary antibodies before detection using chemiluminescence. Signal and quantitation of immunodetected proteins were generated automatically at the end of the run.
MSD assays
Total AKT, GSK-3ß, p70S6K and pp70S6K(Thr-389), pGSK-3ß (Ser-9), pAKT(Ser-473) and pAKT(Thr-308) were quantified from 20μg of total protein from cell lines and mouse tissues on MSD MULTI-ARRAY Assays K15133D-1, K15177D-1 and K151DYD-1 respectively.
RNA extraction, cDNA synthesis and expression assays
Total RNA from frozen mouse tissues and/or Hepa1-6, C2C12 and 3T3-L1 cells were extracted using an RNeasy Plus mini-kit (Qiagen). Quantitative RT-PCR using the TaqMan system (ABI Prism 7700) was carried out using cDNA generated by Superscript III enzyme (Invitrogen) and gene expression normalised relative to the expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Taqman Probes (Table S1) were purchased from Applied Biosystems.
Histology
After exsanguination, pancreatic tissue was dissected, fixed in neutral buffered formaldehyde (Surgipath Europe Ltd, UK) and longitudinally mounted in wax. Sections were cut and stained with Hematoxylin and Eosin (H&E).
Results
Identification of IGT10
Mouse line IGT10 was identified with impaired glucose tolerance from an ENU phenotype driven screen sensitised by haploinsufficiency of the insulin receptor (IR, leading to insulin resistance but not diabetes) as previously described (9) (Figure S1A). The F1 (C57BL/6J (ENU harboring) x C3H/HeH) male founder was backcrossed (BC) to C3H/HeH mice and a cohort segregating both the IR knockout allele and random ENU induced mutations were phenotyped in a glucose tolerance test at 12 and 24 weeks of age. Approximately 40% of the IR heterozygotes showed elevated fasted plasma glucose, elevated insulin and glycosuria (Figure S1B-D).
Mapping and identification of a causative novel splice mutation
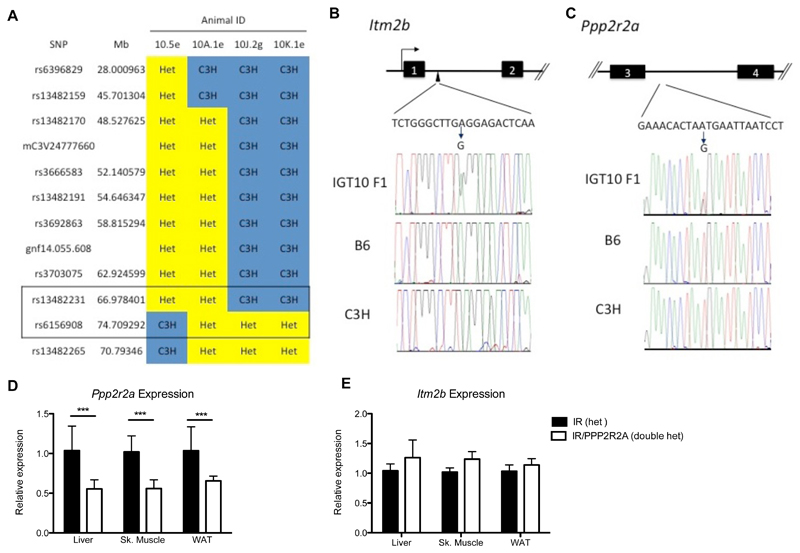
The causative ENU mutation was mapped using SNP genotyping to a 7.7Mb region of chromosome 14 between rs13482231 (66,978,401) and rs6156908 (74,709,292) (Figure 1A). Next-Generation Sequencing (NGS) of this region in DNA from the F1 founder male identified only 2 ENU induced mutations, confirmed by Sanger Sequencing, both of which were non-coding. One was in intron one of the integral membrane protein 2B (Itm2b) gene with no predicted function (73,783,471 A to G) and the second was in intron 3 of Ppp2r2a gene (67,656,803 T to G) predicted to create a new acceptor splice site (Figure 1B and C). Use of the new splice site, was predicted to result in the addition of 8 amino acids followed by a premature stop (Figure S2). To test whether the new splice site was utilized, RNA was extracted from doubly IR/PPP2R2A and IR heterozygous mouse livers and quantitative rtPCR performed utlizing a probe spanning exons 9-10. In doubly heterozygous IR/PPP2R2A mice, 55-65% of the Ppp2r2a transcript was correctly spliced compared to IR only heterozygotes (Figure 1D), and no difference was observed in Itm2b expression (Figure 1E).
Figure 1. Mapping of IGT10 and identification of a causative novel splice mutation.
A) Mapping of minimal candidate region on chromosome 6. B) Confirmation of intronic ENU induced mutation in Itm2b by Sanger Sequencing and diagram of intron/exon structure. C) Confirmation of intronic ENU induced mutation in Ppp2r2a by Sanger Sequencing and diagram of intron/exon structure. D) Reduction in relative expression levels of Ppp2r2a. E) Relative expression levels of Itm2b. QrtPCR data is representative of Mean ± SD, N=8 biological replicates. Statistical analysis 2 way ANOVA * p<0.05, ** p<0.01, *** p<0.001.
Phenotypic characterization
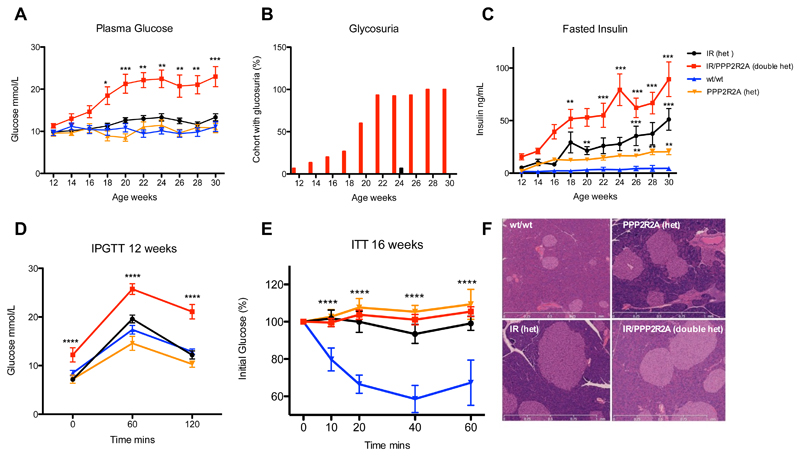
Additional phenotyping cohorts were generated by backcrossing to C3H/HeH. Fasted plasma glucose and insulin levels were measured every 2 weeks and doubly heterozygous IR/PPP2R2A mice showed significantly elevated plasma glucose levels from 18 weeks compared to the other 3 groups (Figure 2A), and the entire group exhibited glycosuria by 28 weeks of age (Figure 2B). Plasma insulin levels were elevated in the IR and PPP2R2A heterozygous mice, and in doubly heterozygous IR/PPP2R2A mice there was a clear additive effect on increased insulin levels, compared to wildtype littermates (Figure 2C). Doubly heterozygous IR/PPP2R2A mice showed significantly impaired glucose tolerance compared to either mutation alone or wildtype littermates at 12 weeks of age (Figure 2D). All three groups compared to the wildtype mice were insulin resistant (Figure 2E); however the doubly heterozygous IR/PPP2R2A mice showed significantly higher starting glucose levels (17.03mmol/L vs 10.89-12.63mmol/L) compared to the other genotype classes. A highly compensatory increase in pancreatic beta cell mass was observed in all three groups compared to wildtypes (Figure 2F). No difference in body weight or food intake was observed in metabolic caging, however double heterozygous IR/PPP2R2A mice drank more and produced more urine as glycosuria developed (Figure S3).
Figure 2. Phenotypic characterization of male IGT10 mice and controls.
A) Fasted plasma glucose levels. B) Presence of glucose in urine. C) Fasted plasma insulin levels. D) IPGTT at 12 weeks of age. E) ITT at 16 weeks of age normalized to starting glucose levels. F) Representative H&E stained pancreas sections. Black circles represent IR heterozygous null mice; Red squares double heterozygous mice, Blue triangles wildtype mice (wt/wt); Orange triangles heterozygous PPP2R2A mice. N=10-15 data expressed as mean ± SD, statistical analysis 2 way ANOVA with a Bonferroni post-test correction for multiple measures * p<0.05, ** p<0.01, *** p<0.001 vs wildtype.
Mis-splicing of Ppp2r2a reduced PPP2R2A and AKT protein levels, insulin-stimulated AKT phosphorylation and downstream signaling
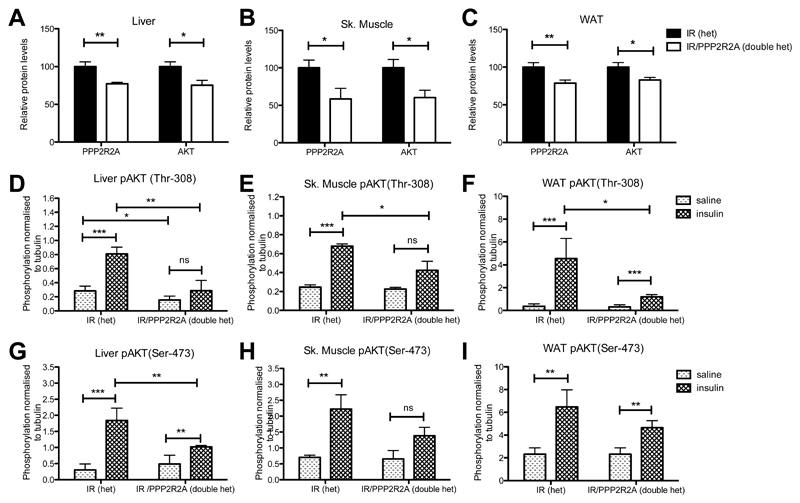
To investigate the effect of mis-splicing of the Ppp2r2a transcript on PPP2R2A protein we extracted protein from doubly heterozygous IR/PPP2R2A or heterozygous IR mice and found significantly reduced PPP2R2A in liver (23% less), skeletal muscle (43%) and white adipose tissue (WAT, 22%) (Figure 3A-C).
Figure 3. Mis-splicing of Ppp2r2a causes a reduction in PPP2R2A and AKT protein levels and a reduction in insulin stimulated AKT phosphorylation.
A) Relative protein concentrations of PPP2R2A and AKT in unchallenged liver lysates. B) Relative protein concentrations of PPP2R2A and AKT in unchallenged skeletal muscle lysates. C) Relative protein concentrations of PPP2R2A and AKT in unchallenged WAT lysates. D-I) Relative protein concentrations of pAKT(Thr-308; D-F), pAKT(Ser-473; G-I), in liver (D,G), skeletal muscle (E,H) and WAT (F,I) isolated from mice exposed to either saline or insulin. Phosphorylated protein quantified by MSD technology and normalized to a parallel sample assayed for Tubulin on the PEGGY system. Light shaded bars saline treatment, dark shaded bars insulin treatment. Data is represented as mean ± SD, N=4 per treatment/per tissue type. Statistical analysis 2 way ANOVA * p<0.05, ** p<0.01, *** p<0.001.
PPP2R2A has been shown to target the PP2A holoenzyme to AKT, which then selectively dephosphorylates Thr-308, returning AKT to the available protein pool (10). However, constitutively phosphorylated AKT is targeted to the proteasome for degradation (11). Consistent with the latter we found a significant reduction in total AKT protein levels in liver (25%), skeletal muscle (40%), and WAT (18%) in doubly heterozygous IR/PPP2R2A mice compared to heterozygous IR mice (Figure 3A-C). Further, we found a significant reduction in the amount of insulin stimulated Thr-308 phosphorylated AKT (normalized to tubulin to account for the differences in total AKT) in liver, skeletal muscle and WAT, in doubly heterozygous IR/PPP2R2A mice that had been fasted overnight and given a bolus of either insulin or saline via the hepatic portal vein (Figure 3D-F). Additionally, we also observed a reduction in Ser- 473 phosphorylation of AKT but this was only statistically significant in liver (Figure 3G-I).
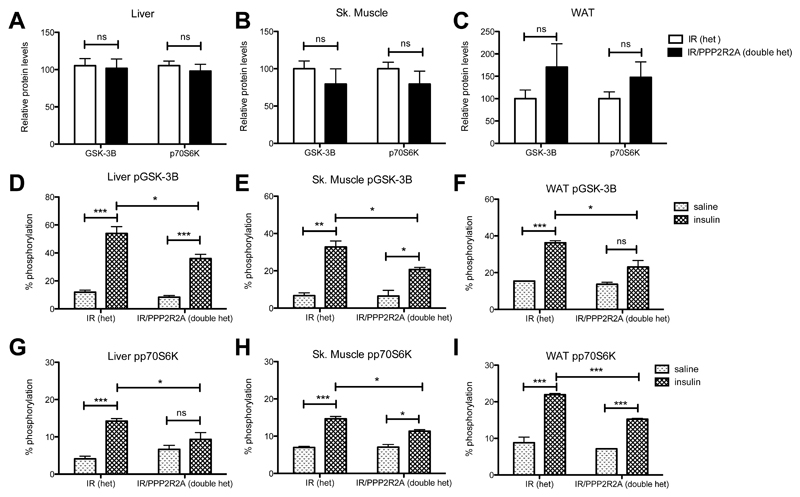
Downstream AKT signaling was assessed by measuring phosphorylation of both glycogen synthase kinase-3 beta (GSK-3ßand ribosomal protein S6 kinase polypeptide 1 (p70S6K), total levels of which were not significantly changed (Figure 4A-C). A significant reduction in the degree of insulin stimulated GSK-3ß and p70S6K phosphorylation was observed in all 3 tissues in doubly heterozygous IR/PPP2R2A mice compared to heterozygous IR mice, (Figure 4D-I).
Figure 4. Mis-splicing of Ppp2r2a causes a reduction in insulin stimulated AKT signaling.
A) Relative protein concentrations of GSK-3ßand p70S6K in unchallenged liver lysates. B) Relative protein concentrations of GSK-3ßand p70S6K in unchallenged skeletal muscle lysates. C) Relative protein concentrations of GSK- 3ßand p70S6K in unchallenged WAT lysates. D-I) Relative protein concentrations of pGSK-3ßD-F), pp70S6K (G-I), in liver (D,G), skeletal muscle (E,H) and WAT (F,I) isolated from mice exposed to either saline or insulin. Phosphorylated and total protein quantified on MSD, data represent percentage phosphorylation. Light shaded bars saline treatment, dark shaded bars insulin treatment. Data is represented as mean± SD, N=4 per treatment/per tissue type. Statistical analysis 2 way ANOVA * p<0.05, ** p<0.01, *** p<0.001.
Knockdown of Ppp2r2a reduced PPP2R2A and AKT protein levels, insulin stimulated AKT phosphorylation and downstream signaling
In order to confirm that the Ppp2r2a mutation is hypomorphic thus resulting in AKT dysregulation, we used siRNAs to knockdown Ppp2r2a expression in hepatocytes (Hepa1-6), myoblasts (C2C12) and adipocytes (3T3-L1). Following knockdown, cells were treated with either insulin or saline (basal), and then protein and RNA was extracted for analysis. 3T3-L1 cells were differentiated into adipocytes prior to siRNA knockdown at day 8 and insulin or saline treated at day10. Differentiation was confirmed by the accumulation of lipid droplets and Fabp4 gene expression (Figure S4). In all cell lines siRNA treatment resulted in significant reduction in Ppp2r2a mRNA levels (Figure S5).
Consistent with the in vivo tissue analysis, there was a significant reduction in total PPP2R2A protein levels; 82.3% reduction Hepa1-6, 68.3% reduction C2C12 and 35.5% reduction 3T3-L1 (Table 1). This resulted in a significant reduction in AKT protein levels; 61.2% reduction Hepa1-6, 21.8% reduction C2C12 and 26.5% reduction 3T3-L1 (Table 1).
Table 1.
Knockdown of Ppp2r2a reduced PPP2R2A and AKT protein levels, insulin stimulated AKT phosphorylation
| Cell line | Relative protein levels PPP2R2A (%) | P-value | Relative protein levels AKT (%) | P-value | Phosphorylation normalized to tubulin | P-value saline vs insulin |
P-value control saline vs saline |
Direction of effect on basal P | P-value control insulin vs insulin |
Direction for stimulation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| saline | insulin | ||||||||||||
| control | Hepa | 100±24 | 100±24.5 | pAKT(thr308) | 2.31±0.76 | 10.78±1.3 | <0.0001 | ||||||
| siRNA1 | 1-6 | 17.55±3.9 | <0.0001 | 42.68±10.6 | <0.0001 | 3.51±0.51 | 6.44±1.78 | 0.003 | 0.009 | ↑ | 0.0007 | ↓ | |
| siRNA3 | 17.8±5.6 | <0.0001 | 34.90±12.3 | <0.0001 | 4.35±0.51 | 4.84±0.49 | ns | 0.0002 | ↑ | <0.0001 | ↓ | ||
| control | Hepa | pAKT(ser473) | 7.23±0.89 | 27.74±3.49 | <0.0001 | ||||||||
| siRNA1 | 1-6 | 4.83±1.35 | 17.31±4.70 | <0.0001 | 0.004 | ↓ | 0.001 | ↓ | |||||
| siRNA3 | 8.73±1.04 | 17.81±1.94 | <0.0001 | 0.02 | ↑ | 0.0001 | ↓ | ||||||
| control | C2C12 | 100±37.5 | 100±30.3 | pAKT(thr308) | 1.19±0.25 | 8.26±2.58 | <0.0001 | ||||||
| siRNA1 | 27.33±6.7 | <0.0001 | 82.19±6.8 | 0.06 | 4.45±0.63 | 6.03±1.31 | 0.02 | <0.0001 | ↑ | ns | ↓ | ||
| siRNA3 | 35.78±20.3 | <0.0001 | 73.22±18.3 | 0.01 | 4.10±0.87 | 5.06±2.09 | ns | <0.0001 | ↑ | 0.03 | ↓ | ||
| control | C2C12 | pAKT(ser473) | 5.06±2.11 | 36.83±11.8 | <0.0001 | ||||||||
| siRNA1 | 7.54±1.44 | 25.40±2.94 | <0.0001 | 0.03 | ↑ | 0.04 | ↓ | ||||||
| siRNA3 | 8.25±0.87 | 19.24±6.99 | 0.003 | 0.006 | ↑ | 0.01 | ↓ | ||||||
| control | 3T3-L1 | 100±18.4 | 104±14.6 | pAKT(thr308) | 0.53±0.19 | 1.98±0.26 | <0.0001 | ||||||
| siRNA1 | 64.63±17.3 | <0.0001 | 77.08±12.3 | <0.0001 | 1.00±0.12 | 1.62±0.91 | ns | 0.0006 | ↑ | ns | |||
| siRNA3 | 65.26±11.4 | <0.0001 | 72.31±8.1 | <0.0001 | 0.79±0.27 | 1.50±0.86 | ns | ns | ns | ||||
| control | 3T3-L1 | pAKT(ser473) | 0.61±0.03 | 2.45±0.39 | <0.0001 | ||||||||
| siRNA1 | 1.99±0.51 | 2.45±0.38 | ns | <0.0001 | ↑ | ns | |||||||
| siRNA3 | 1.10±0.22 | 2.25±0.48 | 0.0003 | 0.0003 | ↑ | ns | |||||||
In siRNA treated Hepa1-6 a small but significant increase in basal Thr-308 phosphorylated AKT (relative to tubulin) was observed and a significant reduction in insulin-stimulated Thr-308 and Ser-473 phosphorylated AKT in Ppp2r2a compared to nonsense siRNA (control) treated cells (Table 1). Similarly, in C2C12 cells there was a significant increase in basal, and a reduction in insulin-stimulated, Thr-308 and Ser- 473 phosphorylated AKT (Table 1). In 3T3-L1 cells, there was a significant increase in basal, but in contrast no difference in insulin-stimulated, Thr-308 and Ser-473 phosphorylated AKT in Ppp2r2a silenced cells compared to control (Table 1).
Next, we examined insulin stimulated AKT signaling in Ppp2r2a silenced cell lines. Under basal saline conditions there was a higher proportion of GSK-3ßphosphorylated in Hepa1-6 and 3T3-L1 Ppp2r2a silenced cells compared to control. However, the proportion of GSK-3ß that was phosphorylated on insulin stimulation was less in Ppp2r2a silenced cells, being largely unresponsive to the effect of insulin compared with control, in hepatocyte (Hepa1-6) and muscle (C2C12) but not in adipocyte (3T3-L1) cells (Table 2).
Table 2.
Knockdown of Ppp2r2a reduced downstream insulin AKT signaling.
| Cell line | Relative protein levels GSK-3β | P-value | Relative protein levels p70S6K | P-value | % Phosphorylation | P-value saline vs insulin |
P-value control saline vs saline |
Direction of effect on basal P | P-value control insulin vs insulin |
Direction for stimulation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| saline | insulin | ||||||||||||
| control | Hepa | 99±6.8 | pGSK-3β | 53.03±7.12 | 107±10.80 | <0.0001 | |||||||
| siRNA1 | 1-6 | 113.92±12.9 | ns | 65.27±7.79 | 61.95±9.16 | ns | 0.01 | ↑ | <0.0001 | ↓ | |||
| siRNA3 | 109.13±13.6 | ns | 69.68±3.29 | 58.09±5.52 | ns | 0.0004 | ↑ | <0.0001 | ↓ | ||||
| control | Hepa | 100±5.5 | pp70S6K | 4.24±0.35 | 10.22±0.73 | <0.0001 | |||||||
| siRNA1 | 1-6 | 109.13±13.6 | ns | 10.58±1.19 | 10.62±1.34 | ns | <0.0001 | ↑↑ | ns | ||||
| siRNA3 | 90.61±17.6 | ns | 9.44±1.79 | 10.49±1.74 | ns | <0.0001 | ns | ||||||
| control | C2C12 | 100±12.45 | pGSK-3β | 59.16±15.65 | 105.54±11.2 | <0.0001 | |||||||
| siRNA1 | 113.94±8.9 | 0.04 | 45.76±3.16 | 56.16±19.59 | ns | ns | 0.0003 | ↓ | |||||
| siRNA3 | 151.30±32.9 | 0.02 | 58.45±14.03 | 69.13±10.40 | ns | ns | 0.0001 | ↓ | |||||
| control | C2C12 | 100±20.5 | pp70S6K | 6.37±2.07 | 12.36±2.33 | 0.0008 | |||||||
| siRNA1 | 129.41±16.2 | 0.02 | 9.71±0.70 | 10.51±0.51 | 0.04 | 0.003 | ↑ | ns | |||||
| siRNA3 | 99.99±6.9 | 0.01 | 10.76±0.85 | 9.92±1.30 | ns | 0.0007 | ↑ | ns | |||||
| control | 3T3-L1 | 100±29.9 | pGSK-3β | 44.47±5.75 | 89.48±2.90 | <0.0001 | |||||||
| siRNA1 | 101.15±33.7 | ns | 72.44±7.40 | 92.36±10.89 | 0.004 | <0.0001 | ↑ | ns | |||||
| siRNA3 | 105.03±27.9 | ns | 65.9±6.24 | 86.16±13.43 | 0.007 | 0.0001 | ↑ | ns | |||||
| control | 3T3-L1 | 99±19.39 | pp70S6K | 4.74±0.81 | 8.23±0.42 | <0.0001 | |||||||
| siRNA1 | 105.39±28.5 | ns | 4.91±0.82 | 5.06±0.41 | ns | ns | <0.0001 | ↓ | |||||
| siRNA3 | 99.99±6.9 | ns | 6.68±0.71 | 7.85±0.638 | 0.01 | 0.001 | ↑ | ns | |||||
A significant increase in the proportion of basal phosphorylation of p70S6K was also observed in all three cell lines after Ppp2r2a knockdown compared to control (Table 2). The proportion of insulin stimulated p70S6K phosphorylation was similar compared to control (Table 2), with a small but significant increase in total GSK-3ßand p70S6K total protein levels was observed in silenced C2C12 cells (both basal and insulin treated), but not in the other cell lines (Table 2).
Impaired transcriptional regulation of hepatic glucose production with Ppp2r2a knockdown
AKT phosphorylates and regulates Forkhead Box O1 (FOXO1) which regulates transcription of gluconeogenic and lipogenic genes. Foxo1 gene expression is increased in the fasted state and reduced after feeding (12), therefore to test whether reduced AKT signaling led to altered regulation of FOXO1 targets we compared serum fed and serum fasted (18 hour) siRNA treated Hepa1-6 cells. Instead of suppression we found Foxo1 mRNA expression was elevated in Ppp2r2a silenced, relative to control siRNA treated cells under serum treatment (Figure 5A).
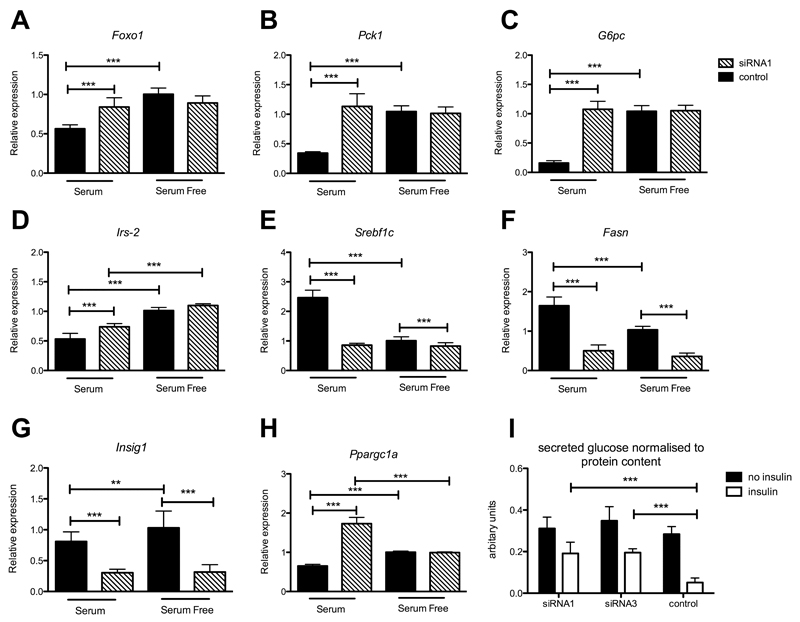
Figure 5. Impaired transcriptional regulation of hepatic glucose production in Hepa 1-6 cells treated with siRNAs targeting Ppp2r2a.
Expression in serum free vs serum fed Hepa 1-6 cells. A) Foxo1, B) Pck1, C) G6pc, D) Irs-2, E) Srebflc, F) Fasn, G) Insig1, H) Ppargc1a. N=6 biological replicates. Data is represented as mean ± SD. Black bars siRNA negative control treatment, hatched bars Ppp2r2a siRNA treatment. I) Glucose production assay on control or siRNA treated Hepa 1-6 cells with insulin (white bars) or without insulin treatment (black bars) N=9 technical replicates (2 independent experiments). Statistical analysis 2 way ANOVA * p<0.05, ** p<0.01, *** p<0.001.
A significant increase in phosphoenolpyruvate carboxykinase (PEPCK (Pck1)) and Glucose-6-phosphatase catalytic subunit (G6pc) and Irs-2 gene expression was observed in Ppp2r2a siRNA treated cells compared to control under serum treatment (Figure 5B-D). The transcription factor Sterol Regulatory Element Binding Transcription Factor 1 (SREBF1) regulates genes required for glucose metabolism, fatty acid and lipid production and we observed a significant reduction in both serum fed and serum free expression of Srebf1c (Figure 5E) in Ppp2r2a siRNA treated cells compared to control. Most strikingly Srebf1c levels were similarly low under both serum and serum free conditions in Ppp2r2a siRNA treated cells. This resulted in a significant reduction in mRNA expression of fatty acid synthase (Fasn) and Insig1 targets of SREBF1, under both sets of conditions (Figure 5F and G). Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (Ppargc1a) a transcriptional coactivator which interacts with and regulates the activities of cAMP response element binding protein (CREB) to drive the transcription of gluconeogenic genes was also significantly upregulated with serum treatment in Ppp2r2a silenced cells compared to control (Figure 5H). To measure the physiological effect of altered gluconeogenesis gene transcription in siRNA silenced cells glucose production assays were performed. As predicted control siRNA treated cells produced significantly less glucose when treated with insulin, however Ppp2r2a siRNA treated cells still secreted significant levels of glucose into the media despite insulin treatment (Figure 5I).
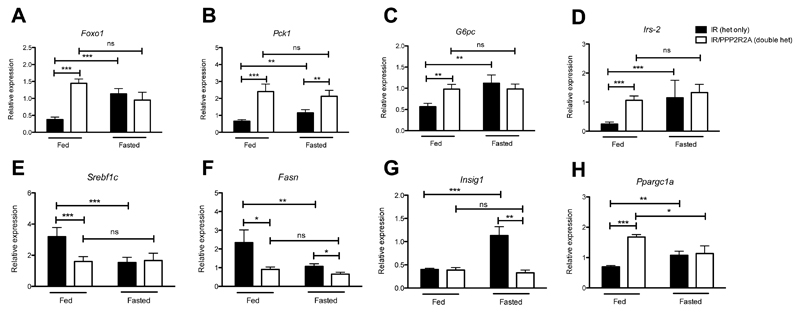
For comparison with the in vivo state, expression analysis was repeated in fed compared to fasted liver cDNA from doubly heterozygous IR/PPP2R2A and heterozygous IR mice. As observed in silenced cells, Foxo1 expression was increased (Figure 6A), with a concomitant significant increase in Pck1, G6pc and Irs-2 gene expression (Figure 6B-D) and a decrease in Srebf1c, Fasn (Figure 6E and F) in doubly heterozygous IR/PPP2R2A fed mice. Levels of Fasn and Insig1 were also significantly reduced in fasted doubly heterozygous IR/PPP2R2A compared to heterozygous IR mice (Figure 6F and G) Ppargc1a was up regulated in doubly heterozygous IR/PPP2R2a compared to heterozygous IR fed mice (Figure 6H).
Figure 6. Impaired transcriptional regulation of hepatic glucose production in livers of PPP2R2A het mice.
Hepatic gene expression in free fed vs 18 hour fasted mice. A) Foxo1, B) Pck1, C) G6pc, D) Irs-2, E) Srebflc, F) Fasn, G) Insig1, H) Ppargc1a. Black bars represent IR het only, white bars IR/PPP2R2A double hets. N=8 biological replicates, data is represented as mean ± SD, statistical analysis 2 way ANOVA compared to IR het only fasted * p<0.05, **p<0.01, ***p<0.001.
Discussion
We have identified a point mutation in intron 3 of the Ppp2r2a gene that resulted in modestly increased fasted insulin and insulin resistance, as did an IR heterozygous knockout mutation. Both mutations together in doubly heterozygous IR/PPP2R2A mice resulted in diabetes; significant hyperglycaemia, hyperinsulinaemia, impaired glucose tolerance and glycosuria. This additive digenic effect of two mutations is similar to mice doubly heterozygous for null alleles of both IR and IRS-1, components of the insulin-signaling pathway, which develop diabetes (4).
The splice mutation reduced Ppp2r2a mRNA levels, although some normal splicing was still retained from the mutant allele, and reduced levels of PPP2R2A protein showing the hypomorphic nature of the mutation. Knockdown studies using siRNAs in insulin responsive cell lines supports the hypothesis that the reduction in PPP2R2A protein levels rather than the production of a truncated dominant negative protein product is responsible for the phenotype. The PP2A holoenzyme consists of a dimeric core enzyme that is composed of a catalytic subunit C, a structural subunit A and a variable regulatory subunit B. There are at least 4 subfamilies of regulatory B subunits (including PPP2R2A) and it is thought that this diversity in regulatory subunits dictates substrate specificity and the subcellular localization of PP2A (13). PPP2R2A is expressed in a wide range of tissues including but not limited to; insulin sensitive tissues (liver, skeletal muscle and adipose tissue) and beta cells of the pancreatic islet (14–16). PP2A is a tumor suppressor and is inactivated or down regulated in colorectal cancer, myeloid leukemia, small cell lung carcinomas and luminal breast cancers (17–22). The role of PPP2R2A in cell growth and division may explain its role as a tumor suppressor, however milder hypomorphic alleles may modulate insulin signaling, in conjunction with other defects, without such a clear alteration of cancer risk.
AKT, a key node in insulin signaling, is phosphorylated by 3-phosphoinositide- dependent kinase-1 (PDK-1) at Thr-308 and mechanistic target of rapamycin (mTOR) at Ser-473 in response to an insulin signal; whereas PPP2R2A containing PP2A holoenzyme specifically dephosphorylate AKT at Thr-308 but not Ser-473 (5; 10). Reduced dephosphorylation of AKT due to reduced PPP2R2A containing PP2A may result in AKT association with C-terminal Hsp70–interacting protein (CHIP), ubiquitination and then degradation (11). This could explain the observed decrease in AKT protein levels that we observed in doubly heterozygous IR/PPP2R2A mice and Pppr2r2a siRNA silenced cell lines. PPP2R2A levels were reduced to lower levels in siRNA treated cells than observed in mouse tissues resulting in a more severe reduction in AKT protein levels and consequently less insulin induced phosphorylation of AKT and its protein targets. Strikingly, in Pppr2r2a siRNA silenced cells we observed significantly increased basal (saline treated serum starved) levels of AKT phosphorylation compared to control, suggesting a significant reduction in PP2A dephosphorylation of AKT in acutely silenced cells. This prolonged active basal AKT state is also reflected in the cell line specific increase in basal phosphorylation of GSK-3ßand p70S6K.
Reduced suppression of hepatic glucose output (HGO) is a hallmark of type 2 diabetes, and HGO is regulated through activation of AKT and phosphorylation of FOXO1 (23–26). Phosphorylation of FOXO1 results in its translocation out of the nucleus, reducing gluconeogenic gene expression (24; 25; 27; 28), and promoting cytoplasmic ubiquitination and degradation (29–31). PPP2R2A containing PP2A holoenzyme has also been shown to specifically dephosphorylate FOXO1 in islet ß-cells under oxidative stress (15). Expression of FoxO1 RNA was increased in serum fed Ppp2r2a silenced cells compared to nonsense treated Hepa 1-6 cells and similarly increased in fed doubly heterozygous IR/PPP2R2A compared to heterozygous IR mice. In contrast in controls, suppression of FoxO1 RNA expression was seen in both serum (compared to serum-free) treated cells, and fed (compared to fasted) liver tissue. Consistent with elevated FoxO1 expression in mutant mice or cells, there was increased RNA expression of its gluconeogenic target genes Pck1 (Pepck), Ppargc1a (Pgc1a) and Gp6c, and another FoxO1 target Irs2, in both cells and tissues. This could lead to a failure of insulin to suppress hepatic glucose output and likely explains a large proportion of the observed phenotype of the mice.
p70S6K is activated in the insulin-signaling pathway via AKT phosphorylation of mTOR, which in turn phosphorylates and activates p70S6K. A well defined negative signaling pathway has been described involving negative phosphorylation of IRS-1 by p70S6K, leading to insulin induced degradation of IRS-1 (32; 33). Insulin stimulated p70S6K phosphorylation levels were significantly reduced in liver, skeletal muscle and WAT in doubly heterozygous IR/PPP2R2A mice. However, in Ppp2r2a siRNA silenced cell lines, which showed a greater reduction in PPP2R2A protein levels than observed in IGT10 mice, a significant increase in basal p70S6K phosphorylation was seen which could result in reduced IRS-1 activity. This was not apparent in insulin treated cells where levels were similar to controls. Therefore, although basal dysregulation could contribute to insulin resistance at least in cell lines, where knockdown is acute, it seems unlikely to be the main explanation for the in vivo phenotypes.
The insulin-regulated activity of AKT regulates SREBP1c activity, which is the master regulator of the transcription of key lipogenic genes (6). Consistent with reduced insulin-stimulated AKT signaling, Srebf1c expression was reduced in serum fed Ppp2r2a silenced cells compared to nonsense treated Hepa1-6 cells and similarly decreased in fed doubly heterozygous IR/PPP2R2A compared to heterozygous IR mice. Consequently, Fasn and Insig1, two SREBP1c regulated genes, were also down regulated under these conditions, therefore disrupting lipogenesis under fed conditions.
We found a reduced proportion of phosphorylated GSK-3ßin response to insulin stimulation, in both tissues from doubly heterozygous IR/PPP2R2A compared to heterozygous IR mice and in corresponding Hepa1-6 and C2C12 Ppp2r2a silenced cells compared to controls, clearly showing impaired insulin signaling. As unphosphorylated active GSK-3ß is a negative regulator of glycogen synthase there is likely to be less glycogen storage of glucose increasing the supply of glucose into the blood and so contributing to the phenotype (34).
Whilst PPP2R2A has not previously been linked to type 2 diabetes in humans PP1A proteins have also been shown to have an important role in glucose metabolism via its regulatory effects on glycogen metabolizing enzymes, including Glycogen synthase (GS), GP and Glycogen phosphorylase kinase (GP Kinase). Heterozygous KO of PPP1R3C results in reduction of glycogen stores, progressive glucose intolerance, hyperinsulineamia and insulin resistance (35). PPP1R3A regulatory subunit of PP1 which binds to muscle glycogen enhancing the dephosphorylation of glycogen bound substrates for PP1 such as GS and GP kinase, which plays an important role in glycogen synthesis but is not essential for insulin activation of glycogen synthase (36). Recently the PP2A subunit PPP2R5C has been shown to couple hepatic glucose and lipid homeostasis, with hepatic knockdown in mice resulting in improved systemic glucose tolerance and insulin sensitivity (37). Such evidence suggests that negative control in the insulin signaling pathway by PP1A and PP2A may prove to be important in susceptibility to type 2 diabetes in humans. Further, population based GWAS studies show that alteration of gene expression of GWAS genes in specific tissues leads to increased diabetes risk and our model is similar in that a hypomorphic allele of a PPP2R2A leads to diabetes in conjunction with insulin resistance.
Supplementary Material
Acknowledgements
The authors would like to thank the Mary Lyon Centre (Harwell, UK) for excellent mouse husbandry.
Funding. MG, YB, RDC, CTE and HH were funded by the Medical Research Council, UK (MRC grant code: MC_U142661184). The gene identification aspect of this research was a collaboration between Amgen Inc. and Harwell. Amgen also provided funding to RDC for these activities.
Footnotes
Duality of interest. All Amgen authors are employees of Amgen Inc and have no conflict/duality of interest. All Medical Research Council authors declare they have no conflict of interest.
Author contributions. MG contributed to the design and carried out the experimental work and preparation of the manuscript. YB contributed to the technical set up of the siRNA experiments. CL carried out the DNA-seq library construction and NGS sequencing, HG carried out the NGS data analysis and identification of ENU candidate hits, WB validated the ENU candidates via Sanger sequencing. HH and CTE ran the samples for the PEGGY experiments. DB and EL carried out all the subsequent genotyping for Ppp2r2a and Itm2b mutations, TJ team leader overseeing ENU/NGS efforts at Amgen. MMV, DJL and RDC contributed to experimental design and manuscript preparation. MG and RDC are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Savage DB, Agostini M, Barroso I, Gurnell M, Luan J, Meirhaeghe A, Harding AH, Ihrke G, Rajanayagam O, Soos MA, George S, et al. Digenic inheritance of severe insulin resistance in a human pedigree. Nat Genet. 2002;31:379–384. doi: 10.1038/ng926. [DOI] [PubMed] [Google Scholar]
- 2.Suliman SG, Stanik J, McCulloch LJ, Wilson N, Edghill EL, Misovicova N, Gasperikova D, Sandrikova V, Elliott KS, Barak L, Ellard S, et al. Severe insulin resistance and intrauterine growth deficiency associated with haploinsufficiency for INSR and CHN2: new insights into synergistic pathways involved in growth and metabolism. Diabetes. 2009;58:2954–2961. doi: 10.2337/db09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar RK, Ellard S, Standiford D, Pihoker C, Gilliam LK, Hattersley A, Dolan LM. Digenic heterozygous HNF1A and HNF4A mutations in two siblings with childhood-onset diabetes. Pediatr Diabetes. 2013;14:535–538. doi: 10.1111/pedi.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Bio. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, Iskandar K, Suga K, Lombes M, Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 8.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 9.Goldsworthy M, Hugill A, Freeman H, Horner E, Shimomura K, Bogani D, Pieles G, Mijat V, Arkell R, Bhattacharya S, Ashcroft FM, Cox RD. Role of the transcription factor sox4 in insulin secretion and impaired glucose tolerance. Diabetes. 2008;57:2234–2244. doi: 10.2337/db07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 11.Su CH, Lan KH, Li CP, Chao Y, Lin HC, Lee SD, Lee WP. Phosphorylation accelerates geldanamycin-induced Akt degradation. Arch Biochem Biophys. 2013;536:6–11. doi: 10.1016/j.abb.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Wondisford AR, Xiong L, Chang E, Meng S, Meyers DJ, Li M, Cole PA, He L. Control of Foxo1 gene expression by co-activator P300. J Biol Chem. 2014;289:4326–4333. doi: 10.1074/jbc.M113.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jangati GR, Veluthakal R, Kowluru A. siRNA-mediated depletion of endogenous protein phosphatase 2Acalpha markedly attenuates ceramide- activated protein phosphatase activity in insulin-secreting INS-832/13 cells. Biochem Biophys Res Commun. 2006;348:649–652. doi: 10.1016/j.bbrc.2006.07.100. [DOI] [PubMed] [Google Scholar]
- 15.Yan L, Guo S, Brault M, Harmon J, Robertson RP, Hamid R, Stein R, Yang E. The B55alpha-containing PP2A holoenzyme dephosphorylates FOXO1 in islet beta-cells under oxidative stress. Biochem J. 2012;444:239–247. doi: 10.1042/BJ20111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora DK, Machhadieh B, Matti A, Wadzinski BE, Ramanadham S, Kowluru A. High glucose exposure promotes activation of protein phosphatase 2A in rodent islets and INS-1 832/13 beta-cells by increasing the posttranslational carboxylmethylation of its catalytic subunit. Endocrinology. 2014;155:380–391. doi: 10.1210/en.2013-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cristobal I, Manso R, Rincon R, Carames C, Senin C, Borrero A, Martinez-Useros J, Rodriguez M, Zazo S, Aguilera O, Madoz-Gurpide J, Rojo F, Garcia-Foncillas J. PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol Cancer Ther. 2014;13:938–947. doi: 10.1158/1535-7163.MCT-13-0150. [DOI] [PubMed] [Google Scholar]
- 18.Ruvolo PP, Ruvolo VR, Jacamo R, Burks JK, Zeng Z, Duvvuri SR, Zhou L, Qiu Y, Coombes KR, Zhang N, Yoo SY, et al. The protein phosphatase 2A regulatory subunit B55alpha is a modulator of signaling and microRNA expression in acute myeloid leukemia cells. Biochim Biophys Acta. 2014;1843:1969–1977. doi: 10.1016/j.bbamcr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalev P, Simicek M, Vazquez I, Munck S, Chen L, Soin T, Danda N, Chen W, Sablina A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res. 2012;72:6414–6424. doi: 10.1158/0008-5472.CAN-12-1667. [DOI] [PubMed] [Google Scholar]
- 20.Dupont WD, Breyer JP, Bradley KM, Schuyler PA, Plummer WD, Sanders ME, Page DL, Smith JR. Protein phosphatase 2A subunit gene haplotypes and proliferative breast disease modify breast cancer risk. Cancer. 2010;116:8–19. doi: 10.1002/cncr.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Graf S, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beca F, Pereira M, Cameselle-Teijeiro JF, Martins D, Schmitt F. Altered PPP2R2A and Cyclin D1 expression defines a subgroup of aggressive luminal-like breast cancer. BMC Cancer. 2015;15:285. doi: 10.1186/s12885-015-1266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, Fukushima Y, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003;52:2905–2913. doi: 10.2337/diabetes.52.12.2905. [DOI] [PubMed] [Google Scholar]
- 24.Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nature genetics. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6:7078. doi: 10.1038/ncomms8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 29.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 31.Nakae J, Kitamura T, Ogawa W, Kasuga M, Accili D. Insulin regulation of gene expression through the forkhead transcription factor Foxo1 (Fkhr) requires kinases distinct from Akt. Biochemistry. 2001;40:11768–11776. doi: 10.1021/bi015532m. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 33.Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050–5062. doi: 10.1128/MCB.21.15.5050-5062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol. 2013;86:191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Lanner C, Kim JH, Vilardo PG, Zhang H, Yang J, Cooper LD, Steele M, Kennedy A, Bock CB, Scrimgeour A, et al. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol Cell Biol. 2001;21:2683–2694. doi: 10.1128/MCB.21.8.2683-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng YS, Seibert O, Kloting N, Dietrich A, Strassburger K, Fernandez-Veledo S, Vendrell JJ, Zorzano A, Bluher M, Herzig S, Berriel Diaz M, et al. PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis. PLoS Genet. 2015;11:e1005561. doi: 10.1371/journal.pgen.1005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.