Abstract
Purpose
Pentaerythritol tetrakis (3,5-di-tert-butyl-4-hydroxyhydrocinnamate) (PTTC) is a cinnamate tetraester with proteasome inhibitor activity, which may be used as a topical treatment in psoriasis, but has a computed log P of 23. The objective of this in vitro study was to determine the intradermal delivery, skin irritation and potential efficacy of PTTC in treating psoriasis.
Methods
Solubility studies were performed to find a suitable vehicle for PTTC. Permeation studies were performed with microneedle-treated skin. A cell culture irritation test was dosed with a positive control, negative control and PTTC. An MTT assay was performed to evaluate cell viability and irritancy. Psoriatic cell culture was also dosed with PTTC and IL-6 levels were determined by ELISA.
Results
Solubility was greatest in dimethyl sulfoxide and ethyl pyruvate, with dimethyl sulfoxide delivering a greater amount (2343.41 ± 384.26 μg) into stratum corneum. PTTC alone as well as topical PTTC emulsion formulation were found to be non-irritant with cell viability of 69.0 ± 5.64% and 74.6 ± 5.03%, respectively. Treatment with neat PTTC slightly reduced IL-6 levels and PTTC emulsion significantly reduced IL-6 levels to 92.53 ± 12.74 pg/ml compared to basal levels (141.69 ± 8.41 pg/ml).
Conclusion
PTTC can be delivered intradermally to potentially treat psoriasis.
Keywords: Dermal delivery, microneedles, skin irritation
Introduction
Psoriasis is a chronic skin condition characterized by increased epidermal cell proliferation and formation of scaly patches. Current treatment options include topical glucocorticoids which offer symptomatic relief, but are associated with side effects, such as skin atrophy and flaring of symptoms with prolonged use [1,2]. Therefore, alternative topical compounds which provide targeted delivery into the skin and a good safety profile are of interest. Arbiser et al. [3] previously reported that compounds isolated from mate tea (Ilex paraguayensis) extracts with cinnamate esters have proteasome inhibitor activity in vitro. Proteasome inhibitors are drugs, which block proteases and thereby prevent breakdown of essential intracellular proteins. A small clinical study conducted in psoriasis and healthy patients found deregulation of the proteasome pathway associated with psoriasis [4]. 3,5-Di-tert-butyl-4-hyroxyhydrocinnamate (PTTC) is a cinnamate tetraester, which is widely used in the rubbers and adhesives industry as an antioxidant with low oral and dermal toxicity. Based on the structure of PTTC and its proteasome inhibitor activity in vitro, we hypothesized this compound may be beneficial in the treatment of psoriasis.
The site of action for PTTC is in the viable epidermis. However, the development of a suitable vehicle for the topical application of PTTC is challenging due to its physicochemical properties. Protopic® is a topical product with the active ingredient tacrolimus, a macrolide immunosuppressant used in the treatment of atopic dermatitis. Tacrolimus is known to have poor dermal penetration and studies have reported the use of microemulsions and nanoparticles to improve targeted delivery [5,6]. PTTC is a highly lipophilic compound with a computed log P of 23.0 and molecular weight of 1117.63 g/mol. Ideal drug candidates for topical delivery have a log P of 1.0–3.0 and molecular weight below 500 g/mol [7]. These properties allow for drugs to permeate across the stratum corneum barrier and into the deeper layers of the skin. The objective of this in vitro study was to determine the intradermal delivery, skin irritation and potential efficacy of PTTC in treating psoriasis.
Materials and methods
Chemicals
PTTC was provided by Accuitis Pharmaceuticals, Inc. (Cumming, GA). Isoamyl alcohol, propylene carbonate, ethyl pyruvate, diethanolamine and n-butanol were generously provided by Jack Aribser (Emory University, Atlanta, GA). HPLC grade solvents were purchased from Fisher Scientific (Pittsburgh, PA).
Solubility testing
Excess amount of PTTC was added to various solvents in scintillation vials. The vials were placed on a shaker at 200 rpm for 24 h. Solutions were filtered and analyzed by HPLC after appropriate dilution. Solubility was determined in dimethyl sulfoxide, ethanol, n-butanol, isoamyl alcohol, propylene carbonate, ethyl pyruvate and triglyceride of captic acid.
Skin preparation
Human dermatomed skin was obtained from a tissue bank and stored at −80 °C. Prior to permeation studies, sealed human skin packets were thawed and after thawing, skin was cut into appropriately sized pieces for permeation.
In vitro permeation studies
Vertical static Franz-type diffusion cells (PermeGear, Hellertown, PA) were used for the permeation studies. The recirculating water bath system was maintained at 37 °C to bring the skin surface temperature to 32 °C. Due to the very poor solubility of PTTC in traditional aqueous receptor media and since only intradermal delivery was desired, a modified method without receptor solution was used to carry out the permeation study. The receptor compartment was covered with aluminum foil and skin was mounted with the stratum corneum side facing up. The skin pieces were equilibrated for 15 min. In the donor compartment, 100 μL of near saturation solution of PTTC in solvent or 100 μL of PTTC cream formulation was added. Skin was dismounted from the Franz cell following 18 h of permeation. Excess donor formulation remaining on the skin was wiped three times with Q-tips soaked in acetonitrile, followed by three times with dry Q-tips. The epidermis was carefully removed with forceps and placed in a scintillation vial with 2 ml of acetonitrile and placed on a shaker at 100 rpm overnight for extraction. For assessment of drug content in the stratum corneum, skin was dismounted from the Franz cell and the tape stripping method was used. An adhesive tape (3 M) was applied onto the skin by rolling a glass rod to allow for good contact. Then a forcep was used to remove the tape and the tape was placed in a 6-well plate with 2 ml of acetonitrile and placed on a shaker at 100 rpm overnight for extraction. A total of 20 tape strips were used to assay the stratum corneum. The remaining stripped skin was minced and extracted with the same method. The extracts were analyzed for drug content by HPLC.
Emulsion formulation
An oil-in-water emulsion containing 30% oil phase and 70% aqueous phase was formulated for application onto micro-needle-treated skin. PTTC was dissolved into triacetin, which served as the oil phase. The aqueous phase consisted of 10% tween 80:span 20 (72:28) in deionized water. For microneedle poration, maltose microneedles (3 × 3 array) were pressed into the skin for 1 min to allow for dissolution and formation of the microchannels. The emulsion (100 μL) was applied onto the porated skin and a permeation study was performed.
HPLC assay
HPLC analysis was carried out on Alliance HPLC Waters 2695 Separations Module attached to a Waters UV detector (Milford, MA). The column was Waters μ Bondapack 10 μm 300 mm × 3.9 mm. The HPLC assay was performed using a gradient method with acetonitrile and water and flow rate of 1.5 ml/min. The gradient was as follows: 90–100% acetonitrile over 5 min, hold till 15 min, 90% till 25 min. Wavelength of detection was set at 210 nm.
Skin irritation testing
A 3D cell culture model of human keratinocytes was purchased from MatTek Corporation (Ashland, MA). Irritation testing was performed according to manufacturer’s protocol. Briefly, upon arrival of the kit, fresh media was replaced and tissue inserts were incubated overnight at 37 °C with 5% CO2. The next day, tissues were dosed with 30 μL of saline (negative control), 30 μL of 5% sodium dodecyl sulfate (positive control), 25 mg of PTTC, and 30 μL of PTTC emulsion (n = 3 for each group). After incubating for one hour, the surface of the tissues was washed thoroughly with saline solution to remove any residual solution. The tissue inserts were incubated again for approximately 24 h. MTT reagent was added and allowed to incubate for 3 h. Absorbance was measured at 340 nm. Cell viability was calculated using a spreadsheet provided by MatTek; viability less than 50% was determined to be irritant.
IL-6 Determination
Psoriatic cell culture kit was purchased from MatTek and manufacturer’s instructions were followed. Briefly, upon arrival of the kit, fresh media was replaced and tissue inserts were incubated overnight at 37 °C with 5% CO2. The next day, tissues were dosed with 30 μL of PTTC emulsion, 30 μL of saline (negative control), or 25 mg of PTTC (n = 3 for each group). Tissues were placed in the incubator; every other day the tissue surface was rinsed with saline, re-dosed, and fresh media was replaced. The tissues were dosed for a total of 96 h, cell culture media was collected and levels of IL-6 were analyzed using ELISA (Fisher Scientific, Pittsburgh, PA).
Statistical analysis
Statistical analysis for multiple groups was carried out using single factor one-way ANOVA. Tukey’s test was performed to determine significant difference between the groups. Statistical analysis for two groups was carried out using t-test. A 0.05 level of probability (p<0.05) was taken as the level of significance.
Results
Solubility and preliminary permeation study
The intradermal delivery of PTTC is challenging due to its very high log P of 23.0 and molecular weight of 1117.63 g/mol. Table 1 shows the solubility of PTTC in various solvents. Based on this initial screening, EP (21.37 ± 1.44 mg/ml) and DMSO (74.44 ± 1.39 mg/ml) had the highest solubility. A preliminary permeation study was conducted utilizing these two solvents due to their ability to solubilize PTTC. The results shown in Figure 1 indicate that penetration of PTTC was significantly greater with DMSO (2343.41 ± 384.26 μg) as a vehicle compared to EP (1263.83 ± 58.15 μg). However, skin penetration was limited to the stratum corneum, the outermost layer of skin, and no detectable drug level was observed in the stripped skin.
Table 1.
Solubility of PTTC in various solvents.
| Solvent | Solubility (mg/ml) | FDA IIG |
|---|---|---|
| Dimethyl sulfoxide | 74.44 ± 1.39 | Yes |
| Ethyl pyruvate | 21.37 ± 1.44 | No |
| Capric triglyceride | 6.49 ± 1.67 | Yes |
| Propylene carbonate | 4.06 ± 0.23 | Yes |
| Triacetin | 2.49 ± 0.79 | Yes |
| Isoamyl alcohol | 2.31 ± 0.56 | No |
| Ethanol | 1.66 ± 0.14 | Yes |
| N-Butanol | 0.87 ± 0.14 | No |
Solubility of PTTC was determined in a series of solvents. The drug had greatest solubility in dimethyl sulfoxide and ethyl pyruvate. All values represent mean ± standard deviation.
Figure 1.
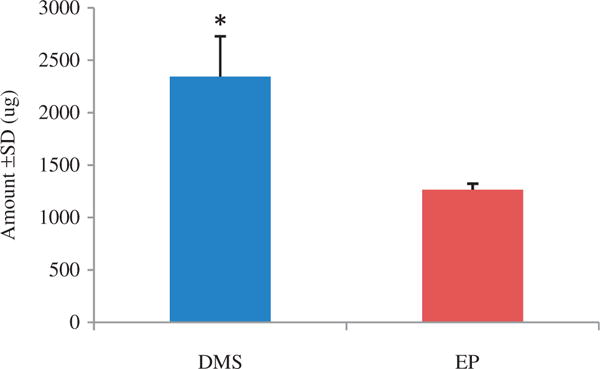
Comparison of PTTC levels in stratum corneum. Skin permeation of PTTC was tested in two vehicles at the same level of saturation. Permeation was limited to the stratum corneum, and no detectable levels of PTTC were found in the stripped skin. All values represent mean ± standard deviation (* indicates p value <0.05). DMSO, dimethyl sulfoxide; EP, ethyl pyruvate.
Intradermal delivery with microneedle approach
Microneedles were used as an approach to aid the permeation of PTTC deeper into the skin. Figure 2 shows that the use of an o/w vehicle to carry PTTC through aqueous microchannels resulted in delivery to the epidermis and dermis. A significantly greater amount of drug was delivered into the dermis (73.91 ± 22.65 ng) compared to the epidermis (20.62 ± 18.00 ng).
Figure 2.
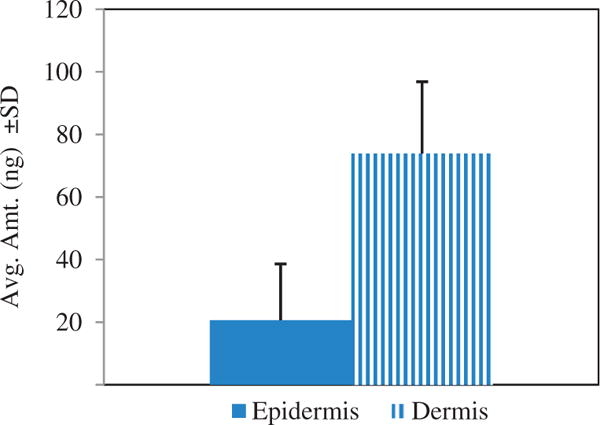
Intradermal delivery of PTTC following microneedle application. An oil-in water emulsion of PTTC was applied to microneedle treated skin. Drug was detected in the epidermis and dermis. All values represent mean ± standard deviation (p value <0.05).
Assessment of skin irritation
An irritation study using a 3D cell culture of human keratinocytes was performed. The results in Figure 3 show that PTTC alone as well as topical PTTC emulsion formulation were found to be non-irritant with cell viability of 69.0 ± 5.64% and 74.6 ± 5.03%, respectively. The positive control, sodium dodecyl sulfate, resulted in only 7.7 ± 0.89% cell viability.
Figure 3.
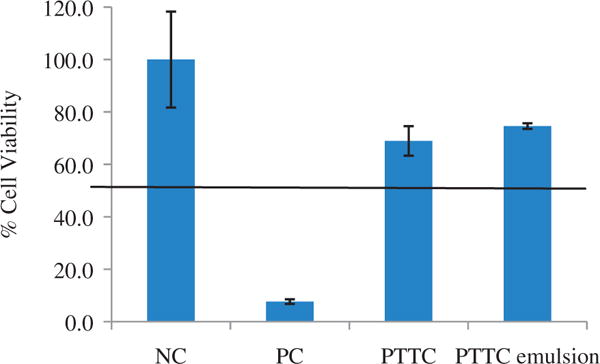
Cell viability following tissue exposure. An in vitro cell culture of human skin was dosed with PTTC and PTTC emulsion. The cell viability was calculated using an MTT assay to determine irritancy. Cell viability greater than 50% indicates a formulation is non-irritant. All values represent mean ± standard deviation (p value <0.05). NC, negative control (saline); PC, positive control (5% sodium dodecyl sulfate).
Reduction in IL-6 levels of psoriatic skin
Finally, a 3D psoriatic cell culture was dosed with PTTC and PTTC emulsion (Figure 4). After 96 h of exposure, cytokine analysis was performed with ELISA. Psoriatic patients have elevated IL-6 levels; therefore, IL-6 was chosen as an endpoint to determine efficacy in this study. The basal level for untreated psoriatic skin was found to be 141.69 ± 8.41 pg/ml. Treatment with neat PTTC slightly reduced IL-6 levels to 122.06 ± 17.59 pg/ml, whereas treatment with PTTC emulsion significantly reduced IL-6 levels to 92.53 ± 12.74 pg/ml. The untreated group was used as a control and had basal levels of 141.69 ± 8.41 pg/ml of IL-6. The PTTC emulsion significantly reduced IL-6 levels to 96 pg/ml.
Figure 4.
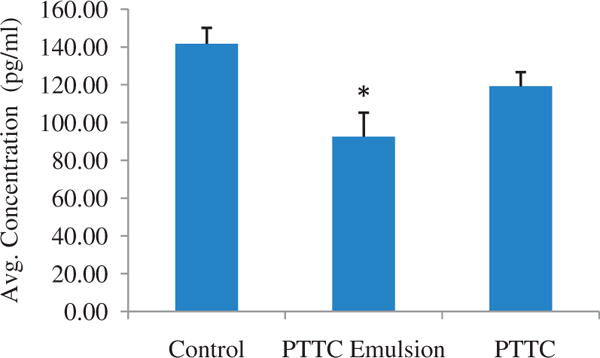
IL-6 levels of treated and untreated psoriatic tissue. An in vitro cell culture of human skin was dosed with PTTC and PTTC emulsion. Levels of IL-6 were determined by ELISA. All values represent mean ± standard deviation (* indicates p value <0.05). Control = saline.
Discussion
Arbiser et al. [3] showed that compounds with cinnamate ester groups display proteosome inhibitor activity, a process which is deregulated in psoriasis [8,9]. PTTC, also referred to as Irganox 1010, is a compound widely used in rubbers as an antioxidant and has four cinnamate ester groups in its structure. Based on the structural elements of PTTC, we hypothesized that this molecule may serve as a potential treatment in psoriasis. The objective of this study was to evaluate the localized skin permeation, irritancy and potential efficacy for this novel psoriasis treatment.
PTTC is a sterically hindered polyphenol, which is soluble in strong non-polar solvents, such as toluene, chloroform and acetone which are not compatible with skin [10]. We performed solubility studies to find a suitable vehicle for PTTC which could be applied topically. Results from Table 1 show that DMSO and EP had the greatest solubility. DMSO is an aprotic solvent which has penetration enhancement properties due to interaction with the lipid domains in human stratum corneum [11]. The current FDA approved topical NSAID solution, Pennsaid®, consists of 45.5% DMSO [12]. A preliminary permeation study of drug dissolved in these vehicles (Figure 1) found that PTTC could penetrate into the stratum corneum of human dermatomed skin. However, despite the solubilization with DMSO, PTTC did not partition out of the stratum corneum and into deeper layers of the skin. No detectable levels of PTTC were found in the underlying stripped skin. This may be attributed to the lipophilicity of the compound, causing PTTC to remain embedded in the lipid matrix of the stratum corneum. The stratum corneum contains approximately 10% of lipid content. According to Figure 1, approximately 1.5 mg of PTTC was delivered into 0.64 cm2 stratum corneum lipids. The high amounts of drug that permeated into the stratum corneum may be a result of the great solubility of PTTC in lipids, especially considering that it has a log P of 23.
Due to the inability of PTTC to partition out of the lipid stratum corneum, microneedles were used as a method to aid delivery to the viable epidermis. Maltose microneedles are 500 μm in length and when pressed into the skin, breach the stratum corneum and dissolve to form aqueous pores which facilitate drug permeation [13]. Hydrophilic molecules are more likely to penetrate through these aqueous channels. Thus, we utilized an oil-in-water emulsion to serve as a vehicle for the lipophilic PTTC to diffuse through micro-channels. The internal oil phase solubilized the drug, while the external water phase allowed for diffusion through the aqueous channels created by microneedles. Microneedles have been extensively studied for enhancement in transdermal delivery. However, research has also been done to show enhancement in local delivery of lidocaine [14] and vaccines [15] into skin. In our study, a significantly greater amount of PTTC permeated into the dermis compared to epidermis as seen in Figure 2. This suggests that the approach can aid in targeted delivery to the viable epidermis. A study with docetaxel, a lipophilic anti-cancer drug with log P of 4.1, loaded into liposomes and applied over microporated skin similarly found enhanced delivery into skin. Given the lipophilicity of PTTC, it is likely that using microneedles would result in accumulation of the drug in skin tissue and minimal diffusion into the blood [16].
The FDA requires that all new topical products be non-irritant to skin [17]. Dermal irritation is the result of a chemical penetrating the stratum corneum and inducing a pro-inflammatory response [18]. A 3D cell culture of human skin keratinocytes has been evaluated by European Center for the Validation of Alternative Methods as an alternative to the rabbit draize test, which is harmful to animals [19]. In our study, both PTTC alone as well as the PTTC emulsion were found to be non-irritant (Figure 3). One concern for evaluation of poorly soluble drugs in vitro is the capacity for these compounds to solubilize and penetrate cell membranes. MatTek suggests 25 mg of neat drug substance be applied on tissues with 100 μL of saline to ensure proper coverage of surface area. However, since PTTC has a log P of 23, triacetin was used as a vehicle to dissolve the neat drug substance, rather than saline. Based on the solubility data obtained, we believe this vehicle will have dissolved enough PTTC to determine an effect on irritation potential. Furthermore, lipophilic drugs can be absorbed transcellularly along cell membranes [20].
Finally, the potential efficacy of PTTC was assessed with a psoriatic cell culture model. The skin penetration study allowed the authors to first determine the feasibility for the molecule to permeate through the skin. The cell culture study was then explored to determine if the molecule could have a biological effect in diseased skin. Psoriatic skin is associated with greater secretion of IL-6, a pro-inflammatory cytokine [8,9]. The cell culture psoriatic model produced by MatTek™ resembles psoriatic skin with hyperproliferation of epidermal cells and overexpression of IL-6 and IL-8. The tissue has a three-dimensional structure similar to that of real skin and comes in a 24-well plate for easy dosing of test material. After dosing the tissue inserts with test material, several endpoints can be selected for investigation, for example, protein analysis, gene expression or cytokine analysis. In this study, we dosed the psoriatic tissue with PTTC for 96 h and measured the levels of IL-6 by ELISA. PTTC emulsion significantly decreased IL-6 compared to the basal levels in the untreated control group (Figure 4). In Figure 1, approximately 100 ng of drug is in equilibrium with the cadaver skin layers, while a higher concentration of drug is in equilibrium with the cell culture. Although the reduction in IL-6 observed is from a relatively higher concentration of drug in the cell culture compared to in vitro skin, it indicates that PTTC has a biological effect which may be beneficial in the treatment of psoriatic skin. In a clinical study with psoriatic patients, treatment with methotrexate downregulated serum IL-6 levels. Since this cytokine is known to be over-expressed in psoriasis, a reversal of this may result in a therapeutic effect, suggesting this type of effect to be positive for psoriasis [21].
In conclusion, we hypothesized that the structural elements of PTTC would make it a viable option for psoriasis treatment. PTTC is a sterically hindered polyphenol with cinnamate ester groups, log P of 23, and molecular weight of 1170 g/mol. With a site of action in the viable epidermis, topical delivery of PTTC is challenging. In this study, microneedles were used to overcome the physicochemical properties and achieve localized skin delivery. Finally, cell culture studies indicated that PTTC was non-irritant and treatment of psoriatic skin resulted in reduced IL-6 levels.
Footnotes
Declaration of interest
This project was funded by Accuitis, Inc.
References
- 1.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 2.Mitra A, Wu Y. Topical delivery for the treatment of psoriasis. Expert Opin Drug Deliv. 2010;7:977–92. doi: 10.1517/17425247.2010.503953. [DOI] [PubMed] [Google Scholar]
- 3.Arbiser JL, Li XC, Hossain CF, et al. Naturally occurring proteasome inhibitors from mate tea (Ilex paraguayensis) serve as models for topical proteasome inhibitors. J Invest Dermatol. 2005;125:207–12. doi: 10.1111/j.0022-202X.2005.23809.x. [DOI] [PubMed] [Google Scholar]
- 4.Henry L, Le Gallic L, Garcin G, et al. Proteolytic activity and expression of the 20S proteasome are increased in psoriasis lesional skin. Br J Dermatol. 2011;165:311–20. doi: 10.1111/j.1365-2133.2011.10447.x. [DOI] [PubMed] [Google Scholar]
- 5.Pople PV, Singh KK. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis. Int J Pharm. 2010;398:165–78. doi: 10.1016/j.ijpharm.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Goebel AS, Neubert RH, Wohlrab J. Dermal targeting of tacrolimus using colloidal carrier systems. Int J Pharm. 2011;404:159–68. doi: 10.1016/j.ijpharm.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 8.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman WA, Levine AD, Massari JV, et al. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BASF. Irganox 1010, Technical Information [online] Basel, Switzerland: 2010. Available from: http://www.telko.com/files/images/telko/ru/basf/termostabilizator/irganox_1010_tds.pdf [last accessed 9 Sep 2015] [Google Scholar]
- 11.Notman R, den Otter WK, Noro MG, et al. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys J. 2007;93:2056–68. doi: 10.1529/biophysj.107.104703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon LS, Grierson LM, Naseer Z, et al. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143:238–45. doi: 10.1016/j.pain.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Kolli CS, Banga AK. Characterization of solid maltose micro-needles and their use for transdermal delivery. Pharm Res. 2008;25:104–13. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- 14.Duan D, Moeckly C, Gysbers J, et al. Enhanced delivery of topically-applied formulations following skin pre-treatment with a hand-applied, plastic microneedle array. Curr Drug Deliv. 2011;8:557–65. doi: 10.2174/156720111796642318. [DOI] [PubMed] [Google Scholar]
- 15.Weniger BG, Glenn GM. Cutaneous vaccination: antigen delivery into or onto the skin. Vaccine. 2013;31:3389–91. doi: 10.1016/j.vaccine.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Gao J, Zhu Q, et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int J Pharm. 2010;402:205–12. doi: 10.1016/j.ijpharm.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration Center for Drug Evaluation and Research Guidance for Industry: Skin irritation and sensitization testing of generic transdermal drug products. Maryland: FDA; 1999. pp. 1–11. [Google Scholar]
- 18.Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116–24. doi: 10.1016/j.clindermatol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Kandarova H, Klausner M, Kubilus J, et al., editors. Update on validation status and industry utilization of normal human 3D (NHU-3D) animal alternative models Presented at 8th World Congress on Alternative and Animal Use. Montreal, Canada: 2011. [Google Scholar]
- 20.Fahr A, van Hoogevest P, May S, et al. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery. Eur J Pharm Sci. 2005;26:251–65. doi: 10.1016/j.ejps.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Elango T, Dayalan H, Subramanian S, et al. Serum interleukin-6 levels in response to methotrexate treatment in psoriatic patients. Clin Chim Acta. 2012;413:1652–6. doi: 10.1016/j.cca.2012.05.007. [DOI] [PubMed] [Google Scholar]


