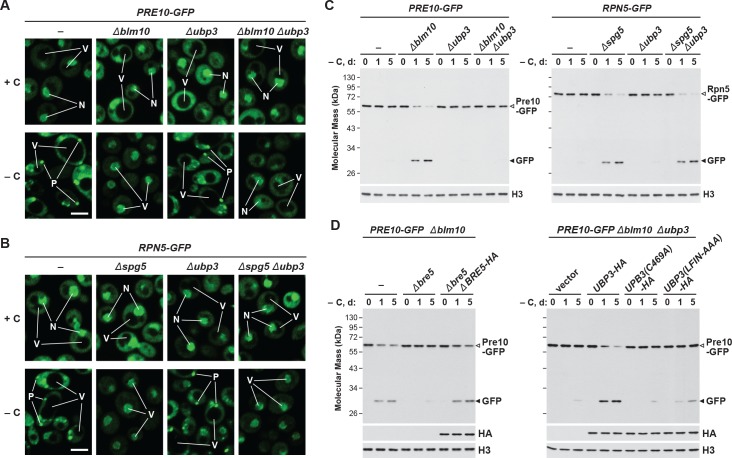
Figure 7. Carbon starvation-induced proteaphagy of the CP in the absence of Blm10 requires the deubiquitylating enzyme Ubp3.
(A and B) Elimination of Ubp3 suppresses transport of the CP (but not the RP) sub-complex to the vacuole in carbon-starved Δblm10 cells. Cells expressing PRE10-GFP (panel A) or RPN5-GFP (panel B) with or without the Δblm10, Δspg5 and/or Δubp3 mutations were grown on nutrient-rich (+N +C) medium and then switched to –C medium for 24 hr before imaging by confocal fluorescence microscopy. N, nucleus; V, vacuole; P, PSG. Scale bar, 2 µm. (C) Accelerated proteaphagy of the CP (but not the RP) in carbon-starved Δblm10 cells is blocked by deletion of Ubp3. PRE10-GFP or RPN5-GFP cells with or without the Δblm10, Δspg5, and/or Δubp3 mutations were switched from +N +C medium to –C medium for the indicated times. Total protein extracts were assayed for GFP release by immunoblot analysis with anti-GFP antibodies, as shown in Figure 1A. Open and closed arrowheads locate the GFP fusion and free GFP, respectively. Immunodetection of histone H3 was used to confirm near equal protein loading. (D) Autophagic degradation of the CP in Δblm10 cells starved for carbon requires active Ubp3 and its co-factor Bre5. PRE10-GFP Δblm10 cells containing the Δbre5 or Δubp3 mutations with or without rescue with HA-tagged Bre5, Ubp3, or mutated versions of Ubp3 lacking the active site cysteine (C469A) or the Bre5 binding site (LFIN-AAAA), were switched from +N +C medium to –C medium for the indicated times and assayed for GFP release by immunoblotting as in panel (C). Accumulation of the Bre5-HA, Ubp3-HA, Ubp3(C469A)-HA and Ubp3(LFIN-AAAA)-HA proteins was confirmed by immunoblotting with anti-HA antibodies.