Fig. 1.
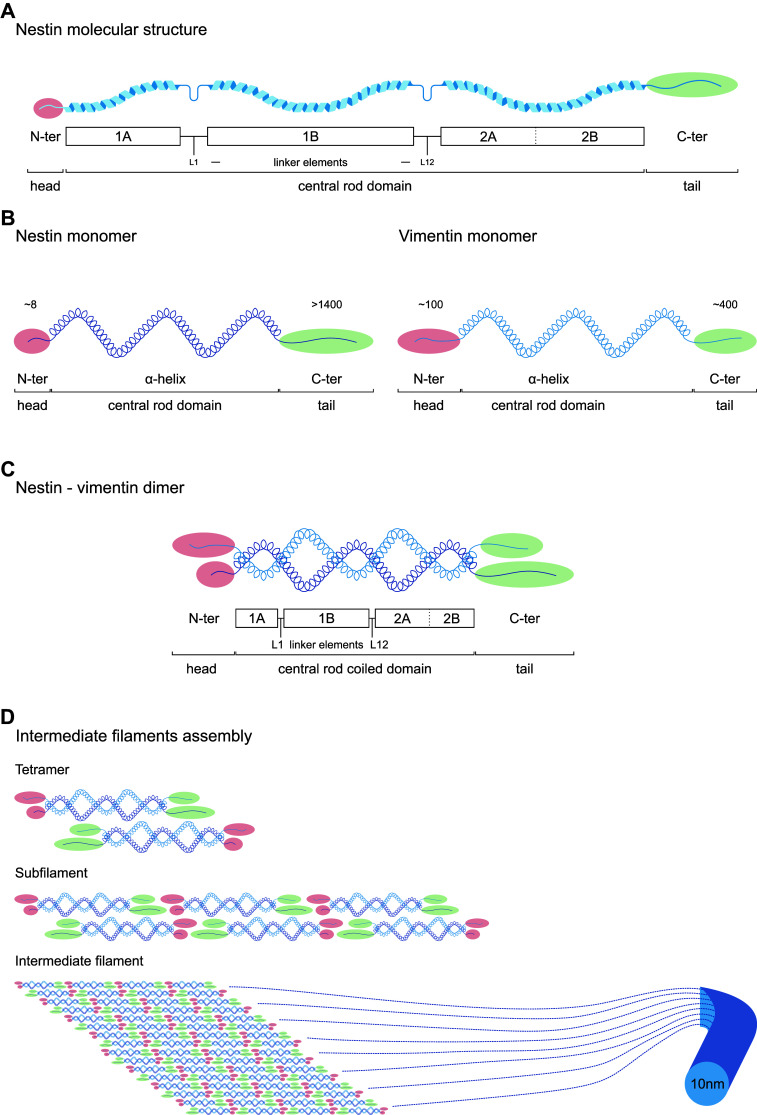
Nestin structure and assembly into intermediate filament. a Molecular structure of Nestin. Nestin shares a common structure with the other intermediate filaments that consists of a central α-helical rod domain (blue) of conserved size, flanked by a globular N-terminus (‘head’, red) and C-terminus (‘tail’, green) domains. The central α-helical rod domain consists of three segments separated by two linkers, i.e., coil 1A, linker 1, coil 1B, linker 12 and coil 2, with 2A and 2B and stutter between them. b Particularities of Nestin monomer. Nestin shows a short N-terminus (≈ 8 amino acids) and a long C-terminus (> 1400 amino acids) end, compared to other intermediate filaments like vimentin (≈ 100 and 400 amino acids, respectively). c Nestin forms heterodimers. N-terminus is required for intermediate filament assembly, and free C-terminus may interact with other cytoskeleton components. The short N-terminus disables Nestin to self-assemble into higher order structures, so Nestin needs other intermediate filaments to assemble, like vimentin. The central rod domain contains hydrophobic repeats (heptad repeats) that mediate dimerization allowing two α-helices to wrap around each other and become a ‘coiled coil’. Therefore, intermediate filament dimer rod central domain is known as central rod coiled domain. d Hypothetical intermediate filament assembly. Two dimers coil in antiparallel way in head-to-tail association to make a tetramer. Assembly takes place through rapid lateral aggregation of eight tetramers to form subfilaments, which assemble axially to form the intermediate filament (diameter ~ 10 nm). Numbers in N-termini and C-termini ends indicate number of amino acid residues. N-ter N-terminus, C-ter C-terminus
