Abstract
Aim
Evaluating the recurrence patterns of high-grade astrocytomas in patients who were treated with radiotherapy (RT) plus temozolomide (TMZ).
Background
The current literature suggests that reducing the margins added to the CTV does not significantly change the risk of recurrence and overall survival; thus, we decided to analyze our data and to examine the possibility of changing the adopted margins.
Materials and methods
From February 2008 till September 2013, 55 patients were treated for high-grade astrocytomas, 20 patients who had been confirmed to have recurrence were selected for the present study. Post-operative MRI was superimposed on the planning CT images in order to correlate the anatomical structures with the treatment targets. Recurrences were defined according to the Response Assessment Criteria for Glioblastoma. The mean margins of the PTVinitial and PTVboost were 1.2 cm and 1.4 cm, respectively. The analysis of the percentage of the recurrence volume (Volrec) within the 100% isodose surface was based on the following criteria: (I) Central: >95% of the Volrec; (II) In-field: 81–95% of the Volrec; (III) Marginal: 20–80% of the Volrec; and (IV) Outside: <20% of the Volrec.
Results
Of the 20 patients, 13 presented with central recurrences, 3 with in-field recurrences, 2 with marginal recurrences and 2 with outside recurrences. Therefore, the lower Volrec within 100% of the prescribed dose was considered in the classification.
Conclusions
Of the selected patients, 80% had ≥81–95% of the Volrec within 100% of the prescribed dose and predominantly had central or in-field recurrences. These results are comparable with those from the literature.
Keywords: High-grade astrocytoma, Brain radiotherapy, Temozolomide, Recurrence patterns, Planning target volume
1. Background
According to the classification of the World Health Organization, anaplastic astrocytoma (AA) and glioblastoma (GBM) correspond to high-grade astrocytomas (grades III and IV, respectively) and are associated with a mean survival of approximately 14 months.1 Treatment with surgery is indicated in most cases and the surgical resection should be as complete as possible. Adjuvant fractionated radiotherapy (RT) is considered a standard treatment for high-grade astrocytomas, which is supported by level 1 evidence. In most cases, the therapy is divided into 30 fractions that are distributed over 6 weeks, with a total dose of 60 Gy.1, 2, 3
International Commission on Radiation Units and Measurements4 recommend for the initial RT for high-grade astrocytoma, two clinical target volumes (CTVs) delineated by magnetic resonance imaging (MRI). In the first phase of treatment, an initial CTV (CTVinitial) is defined that accounts for edema and potential areas of microscopic infiltration in a T2-weighted fluid attenuated inversion recovery (FLAIR) image. In the second phase of treatment, a boost CTV (CTVboost) is defined based on the surgical cavity of the T1-weighted post-operative MRI, after the administration of an intravenous contrast medium. To account for possible intrinsic geometrical errors in the system, a margin is added in all directions, which corresponds to the initial and boost planning target volumes (PTVs).
The best therapeutic results have been obtained when the chemotherapeutic agent temozolomide (TMZ) was administered concomitant/adjuvant to RT.5 The exposure of TMZ reduces the DNA-repair enzyme 06 methylguanine-DNA methyltransferase (MGMT) stepping forward in the long term survival rate of patients.6 A randomized phase III EORTC protocol compared the use of concomitant TMZ to RT alone.7 Concomitant TMZ resulted in an increase in the mean survival from 12.1 to 14.6 months with minimal additional toxicity, and 20% of the patients had a survival rate of up to 36 months. Furthermore, TMZ has been used in recurrent cases due to its being well tolerated and having good oral bioavailability.8
Therapeutic success hinges on a balance between the risks and benefits inherent to treatment. In RT, delineation of the tumor or tumor cavity, and margins that are added to the delineation, are required because of the possible toxicity to healthy tissues adjacent to the lesion. These additional margins should account for the possibility of subclinical disease as well as intrinsic errors in the system, in order to reduce the probability of tumor recurrence.3, 8 The current literature suggests that reducing the margins added to the CTV does not significantly change the risk of recurrence and overall survival.9
2. Aim
In this study, we aimed to retrospectively evaluate the recurrence patterns of high-grade astrocytomas in patients who underwent RT and TMZ when the recurrence was within, near, or outside the adopted margins, and to examine the possibility of either reducing or increasing the adopted margins.
3. Materials and methods
3.1. Patients
From February 2008 till September 2013, 55 patients were treated for high-grade astrocytomas at a RT clinic in Florianópolis-SC Brazil. 20 patients who had been confirmed to have recurrence were selected for the present study. All those patients were more than 18 years of age, underwent RT and concomitant/adjuvant TMZ and presented a follow-up of at least 12 months. Patients who had previous treatments in other centers or who did not present recurrence confirmed by MRI were not selected. This study was approved of the Research Ethics Committee – CEPON – SC (CAAE: 32293814.9.0000.5355).
3.2. Chemo and radiotherapy procedures
The treatment protocol consisted of RT (starting 2–4 weeks after surgical resection of the tumors) plus continuous daily chemotherapy with TMZ (75 mg/m2 of body-surface area/day, 7 days per week from first to the last day of RT) followed by adjuvant TMZ for 6 cycles (150–200 mg/m2/5 days during each 28-day cycle). All patients underwent computed tomography (CT) simulation with a section thickness of 0.625 cm, in the supine position on a scanning table. For delineation, post-operative MRI images were superimposed on the planning CT images in order to correlate the anatomical structures with the treatment target sites.
Delineation of the tumor cavity in each case was performed manually, section by section, by an experienced radiation oncologist, and the T1-weighting after the administration of an intravenous contrast medium was considered. For this volume, a margin corresponding to the PTVboost, or a PTV of 60 Gy was adopted that received the total prescribed dose. We used the margins of post-surgical edema was analyzed in a T2-weighted FLAIR image and the margin accounted for the edema corresponding to the PTVinitial, or a PTV of 46 Gy. In some cases, when the pre-operative MRI results were accessible, a correlation between the tumor resection cavity and the tumor was performed in order to cover areas with possible subclinical disease.
Regarding the adopted treatment modality, the selected patients underwent three-dimensional conformal RT (3DCRT) or intensity modulated RT (IMRT). The sequence was either conventional (one phase of 60 Gy divided into 30 fractions of 2 Gy) or conducted in two stages (the first at 46 Gy of the prescribed dose, divided into 23 fractions of 2 Gy, followed by a 14 Gy boost split into 7 fractions of 2 Gy, totaling 60 Gy).
Orthogonal kV CT images of the patient position were linked to the planning CT through bone fusion. The mean of the results from the first 3 days of treatment was calculated using the deviation indicated by the BrainLab ExacTrac 5.5 system. The isocenter treatment was modified and checked weekly by the imaging software, considering a tolerance of 2 mm.
3.3. Follow-up and verification of tumor recurrence
The MRI controls were performed approximately every 3 months after treatment during the first year and every 6 months during the following years. An experienced radiologist evaluated all MRI results. The protocol used for the MRI displayed small variations by location. Generally, the main T1- and T2-weighted images were submitted after the administration of an intravenous contrast medium that was suitable for MRI. Tumor recurrence was defined according to the Response Assessment Criteria for Glioblastoma.10 Specifically, it was indicated by an increased signal in new areas, a 25% increase in signal in pre-existing areas compared to the initial signal, and an abnormal signal in existing areas without an increase in size. In some cases, brain perfusion and spectroscopy were required to distinguish actual tumor recurrence from tumor pseudoprogression.
The volume of each recurrence identified by MRI was correlated with 100% of the prescribed dose of the treatment plans sum through a dose volume histogram (DVH). The percentage analysis of the recurrence volume within the 100% isodose surface was based on the following criteria: (I) Central: >95% of the recurrence volume (Volrec) within the 100% isodose surface; (II) In-field: 81–95% within the 100% isodose surface; (III) Marginal: 20–80% within the 100% isodose surface; and (IV) Outside: <20% within the 100% isodose surface.
4. Results and discussion
4.1. Patient characteristics
According to the operations descriptions (provided by the neurosurgeons), a complete tumor resection was achieved in 65% of the cases. Additionally, 90% of the cases were GBM and 10% were AA. Both diagnoses were confirmed by biopsy. Since not all of the patients underwent immediate post-operative MRI (within 48 h of surgery), it was decided not to describe the degree of resection based on the images. Of the 20 patients in the study, 18 underwent 3DCRT and 2 underwent IMRT. Forty percent (8 patients) were treated according to the conventional sequence while the others were treated in only one phase. The mean margins of the PTVinitial and the PTVboost were 1.2 cm (0.8–1.7 cm) and 1.4 cm (0.9–1.9 cm), respectively. The mean age of the patients was 56 years. Sixty percent of the patients were male and forty were female. The patient characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| Characteristic | Data | % | |
|---|---|---|---|
| Sex | Male | 12 | 60 |
| Female | 8 | 40 | |
| Age | Average | 56 years | |
| Range | 36–77 years | ||
| Tumor histology | GBMa | 18 | 90 |
| AAb | 2 | 10 | |
| Surgical resection | Complete | 12 | 60 |
| Partial | 6 | 30 | |
| No description | 2 | 10 | |
| RTc | 23 × 200 cGy + 7 × 200 cGy | 8 | 40 |
| 30 × 200 cGy | 12 | 60 | |
| Margin (PTVdinitial) | Average | 1.2 cm | |
| Range | 0.8–1.7 cm | ||
| Margin (PTVboost) | Average | 1.4 cm | |
| Range | 0.9–1.9 cm | ||
GBM: glioblastoma.
AA: anaplastic astrocytoma.
RT: radiotherapy.
PTV: planning target volume.
4.2. Analysis of recurrence
The analysis of the Volrec for all patients was performed as described above, using the plans sum DVH. Of the 20 patients, there were 13 with central, 3 with in-field, 2 with marginal, and 2 with outside recurrences (Table 2). A total of 26 recurrences were observed, which included several patients with multiple recurrences. Therefore, the categorization accounted for the lower Volrec within the 100% dose.
Table 2.
Categorization of the recurrences.
| Category | Number of patients | % |
|---|---|---|
| Central | 13 | 65 |
| In-field | 3 | 15 |
| Marginal | 2 | 10 |
| Outside | 2 | 10 |
Only one recurrence was observed in 85% of the patients. The remaining 15% of the patients had 2–3 recurrences that were observed by MRI. A case of a single recurrence with >95% of the volume within 100% of the prescribed dose is shown in Fig. 1. A case of a single recurrence with approximately 84% of the volume within 100% of the prescribed dose is shown in Fig. 2. A case involving multiple tumor recurrences, one with >95% of the volume within 100% of the prescribed dose and the other with approximately 25% is shown in Fig. 3. Multiple tumor recurrences are shown in Fig. 4, two with >95% of the volume within 100% of the prescribed dose and one with 81% of the volume within 100% of the prescribed dose.
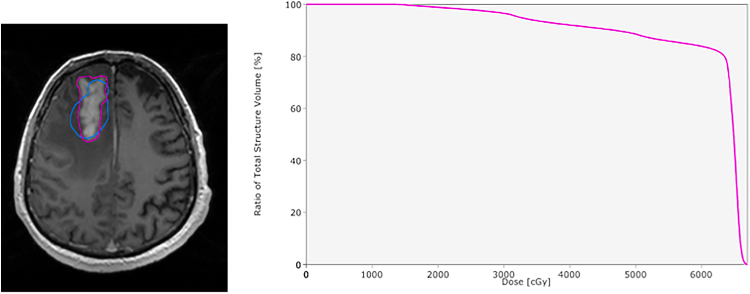
Fig. 1.
A case of a single recurrence with >95% of the volume within 100% of the prescribed dose. In the first one, magnetic resonance imaging of a tumor recurrence. The delineation is shown in green and the planning target volume in pink. In the second one, dose volume histogram showing >95% of the volume of the recurrence within 100% of the prescribed dose.
Fig. 2.
A case of a single recurrence with approximately 84% of the volume within 100% of the prescribed dose. In the first one, magnetic resonance imaging of tumor recurrence with the delineation shown in pink and the planning target volume in blue. In the second one, dose volume histogram showing approximately 84% of the volume of the recurrence within 100% of the prescribed dose.
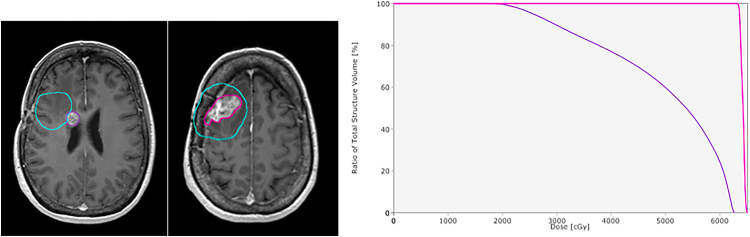
Fig. 3.
A case of multiple tumor recurrences, with two different recurrence volume percentages within 100% of the prescribed dose. In the first one, magnetic resonance imaging of the first and second tumor recurrence. The delineation of first recurrence is shown in purple and the planning target volume (PTV) in blue. The delineation of second recurrence is shown in pink and the PTV in blue. In the second picture, dose volume histogram showing the recurrence percentages. The first shows approximately 25% of the volume of the recurrence within 100% of the prescribed dose and the second has >95%.
Fig. 4.
A case of multiple recurrences with three different percentages of the recurrence volume within 100% of the prescribed dose. In the first one, magnetic resonance imaging of the first tumor recurrence with the delineation in blue and the planning target volume (PTV) in red. After, magnetic resonance imaging of the second recurrence with the delineation in green and the PTV in red. Finally, magnetic resonance imaging of the third recurrence with the delineation in yellow, and the PTV in red. (b) Dose volume histogram showing the recurrence percentages. The first and the last recurrence had >95% of the volume within 100% of the prescribed dose, and the second had approximately 82% of the volume within 100% of the prescribed dose.
We aimed to examine the pattern of recurrence of high-grade astrocytomas after treatment with RT and TMZ in order to categorize them into four groups: central, in-field, marginal, and outside, a system that was originally presented by Lee et al.,3 and has frequently been applied in similar studies. We opted by using the definition of McDonald et al.,10 who analyzed the patterns of recurrence trough the 100% isodose line, instead of Lee et al.3 who preferred the 90% isodose.
Due to the tumor heterogeneity of high-grade astrocytoma, the therapeutic strategy may combine several approaches, which includes surgery, RT, chemotherapy and combined treatment. In these terms, the unique radiosensitizing agent with call level I evidence of benefit is TMZ.11 Milano et al.,12 proposed that TMZ could affect the location of the recurrence thereby establishing a pattern of recurrence, due to its ability to increase tissue radiosensitivity. The study presented possible relationships between the origin of the recurrence and the time between manifestations, stating that the risk of marginal or outside lesions, not likely to be related to the original tumor, was directly proportional to patient survival. Moreover, unlike our study, which showed a greater number of central recurrences, most of the relapses analyzed by Milano et al. were categorized as in-field. However, in both studies, approximately 80% of the recurrences were observed within the irradiated volume, a result also reported by Lee et al.3 Still on the use of TMZ, Stupp et al.,13 in their huge trial with a total of 573 cases from 85 centers, presented a median progression free survival of 6.9 months with RT plus TMZ and 5.0 months with RT alone (p < 0.001 by the log-rank test), suggesting a statistically significant progression free survival benefit with the addition of TMZ to RT.
There is controversy regarding the margin that should be added to the CTV. Comprehensive studies, such as the RTOG 0825, have shown that this margin should be at least 2 cm. However, in some cases, due to organs at risk that are close to the lesion, this recommendation cannot be followed. Therefore, McDonald et al.10 assumed hypothetical margins of 2 and 2.5 cm and compared these to the actual margins, which in some cases were <1 cm, adopted by the group. The results showed no increased risk of marginal or outside recurrence due to a lower PTVboost margin, consistent with the study by Chang et al.14 In contrast, Gaspar et al.,15 showed that a lower PTVboost increased the risk of marginal recurrence. In this study, average PTVboost margins of 1.4 cm were adopted based on the tumor location. Two cases of marginal recurrences were observed in this study. One showed 72% of the Volrec within the isodose line of 100% while the other showed only 25%. In the first case, with a partial resection, the average margin was 1.4 cm and could not be isotropic by reason of the proximity to organs at risk, such as the optic chiasm. Due to the location of the original tumor, edema related to the recurrence observed in the T2 FLAIR, expanded beyond the average margin. Therefore, 28% of the volume was outside the irradiation field. In the second case, 75% of the Volrec was outside the PTVboost, with an average margin of 1.2 cm. Thus, in both cases a greater margin of 2–2.5 cm would not have influenced the categorization of the recurrences.
In terms of the two cases of outside recurrences, the Volrecs were 100% outside of the irradiated volume. Disseminated subependymal disease was observed in one of the cases of recurrences and hippocampal disease was observed in the other. The existence of neural stem cells in the subventricular zone should also be noted. Gene expression in gliomas follows a similar pattern to that observed in neural stem cells. Therefore, the risk of dissemination appears to be greater when the disease is located in proximity to or in the margin of this region.16, 17, 18 Therefore, it can be hypothetically stated that intraventricular resection and non-irradiation of the subventricular zone may increase the risk of outside recurrence, as was observed in this study. Gupta et al.,19 presented positive results for the overall survival of patients who underwent irradiation of potential neoplastic stem cell niches in the subventricular zone. This method has recently been adopted at our center, although no definitive results have been obtained thus far.
A better definition of the treatment target volume and the margins that should be adopted is essential in order to prevent improper irradiation of microscopic disease. Consequently, alternative methods, such as MRI spectroscopy and positron emission tomography/computed tomography (PET/CT), can be useful.20, 21, 22 However, the dissemination pattern of the disease will depend on numerous factors such as the initial location of the tumor and the surgical procedure performed, which directly affects overall patient survival. In a study by Konishi et al.,23 there was a direct correlation between the type of resection and the long-term results. Of the 65 cases that were selected, 23% of the patients underwent resection of more than 98% of the tumor volume and remained disease-free for an average of 17 months. In our study, it was not possible to identify a specific factor that was associated with the predominant recurrence pattern observed. Nonetheless, it was determined that margins of <2 cm did not affect the results obtained for the two marginal cases, as the margins were within the overall average.
5. Conclusions
In this study, 20 patients with high-grade astrocytomas who were treated with RT and TMZ were analyzed. Of the 20 patients, 13 had central, 3 had in-field, 2 had marginal, and 2 had outside recurrences. A total of 26 recurrences were observed. The average margin adopted for the PTVboost was 1.4 cm (0.9–1.9 cm), and had no direct impact on the pattern of tumor recurrence, as 80% of cases had Volrec 81–95% or more within 100% of the prescribed dose. These results are comparable with those from the literature. Therefore, a reduction or increase in the PTVboost would not affect the recurrence patterns.
Conflict of interest
None declared.
Financial disclosure
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support for the realization of this research.
Acknowledgements
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support for the realization of this research.
References
- 1.Schneider T., Mawrin C., Scherlach C., Skalej M., Firsching R. Gliomas in adults. Dtsch Ärztebl Int. 2010;107:799–808. doi: 10.3238/arztebl.2010.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park I., Tamai G., Lee M.C.<ET-Al>. Patterns of recurrence analysis in newly diagnosed glioblastoma multiforme after three-dimensional conformal radiation therapy with respect to pre-radiation therapy magnetic resonance spectroscopic findings. Int J Radiat Oncol Biol Phys. 2007;69:381–389. doi: 10.1016/j.ijrobp.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.W., Fraass B.A., Marsh L.H.<ET-Al>. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 4.Chavaudra J., Bridier A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother. 2001;5:472–478. doi: 10.1016/s1278-3218(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 5.Brandes A.A., Tosoni A., Franceschi E.<ET-Al>. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 6.Anjum K., Shagufta B.I., Abbas S.Q.<ET-Al>. Current status and future therapeutic perspectives of glioblatoma multiforme (GBM) therapy: a review. Biomed Pharmacother. 2017;92:681–689. doi: 10.1016/j.biopha.2017.05.125. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R., Hegi M.E., Mason W.P.<ET-Al>. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Harasaki Y., Waziri A. Potential usefulness of radiosensitizer in glioblastoma. Neurosurg Clin N Am. 2012;23.:429–437. doi: 10.1016/j.nec.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Aydin H., Sillenberg I., von Lieven H. Patterns of failure following CT-based 3-D irradiation for malignant glioma. Strahlenther Onkol. 2001;177:424–431. doi: 10.1007/pl00002424. [DOI] [PubMed] [Google Scholar]
- 10.McDonald M.W., Shu H.K., Curran W.J., Jr., Crocker I.R. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;79:130–136. doi: 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Wen P.Y., Macdonald D.R., Reardon D.A.<ET-Al>. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 12.Milano T.M., Okunieff P., Donatello R.S.<ET-Al>. Patterns and timing of recurrence after temozolomine-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:1147–1155. doi: 10.1016/j.ijrobp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Stupp R., Hegi M.E., Mason W.P.<ET-Al>. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:10. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 14.Chang E.L., Akyurek S., Avalos T.<ET-Al>. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68:144–150. doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Gaspar L.E., Fisher B.J., Macdonald D.R.<ET-Al>. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. doi: 10.1016/0360-3016(92)91021-e. [DOI] [PubMed] [Google Scholar]
- 16.Barami K., Sloan A.E., Rojiani A., Schell M.J., Staller A., Brem S. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009;16:195–201. doi: 10.1016/j.jocn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Barami K. Biology of the subventricular zone in relation to gliomagenesis. J Clin Neurosci. 2007;14:1143–1149. doi: 10.1016/j.jocn.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Gupta T., Nair V., Paul S.N.<ET-Al>. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109:195–203. doi: 10.1007/s11060-012-0887-3. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M.D., Du Plessis D.G., Walker C., Smith T.S. Advanced MRI in the management of adult gliomas. Br J Neurosurg. 2007;21:550–561. doi: 10.1080/02688690701642020. [DOI] [PubMed] [Google Scholar]
- 21.Cha S. Neuroimaging in neuro-oncology. Neurotherapeutics. 2009;6:465–477. doi: 10.1016/j.nurt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essig M., Giesel F., Stieltjes B., Weber M.A. Functional imaging for brain tumors (perfusion, DTI and MR spectroscopy) Radiology. 2007;47:513–519. doi: 10.1007/s00117-007-1518-4. [DOI] [PubMed] [Google Scholar]
- 23.Konishi Y., Muragaki Y., Iseki H., Mitsuhashi N., Okada Y. Patterns of intracranial glioblastoma recurrence after aggressive surgical resection and adjuvant management: retrospective analysis of 43 cases. Neurol Medico-Chir (Tokyo) 2012;52:577–586. doi: 10.2176/nmc.52.577. [DOI] [PubMed] [Google Scholar]