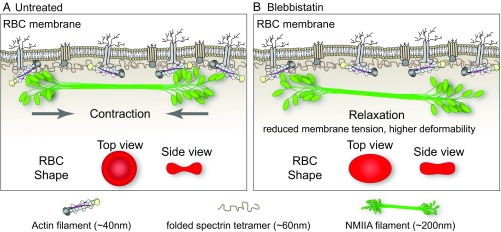
Fig. 6.
Model for NMIIA contractility in the spectrin–F-actin network and its effect on RBC membrane morphology and mechanical properties. (A) Two-dimensional network of long, flexible spectrin tetramers that cross-link short actin filaments (the spectrin–F-actin network) is attached to the plasma membrane (not drawn to scale) via associations of network components with transmembrane proteins. NMIIA bipolar filaments can associate with the short F-actins of the spectrin–F-actin network via their motor domains. Through these interactions, NMIIA contractile forces (arrows) could promote membrane tension in the spectrin–F-actin network to maintain normal biconcave RBC shapes, as shown in top and side views (red RBCs), and to control RBC deformability. The NMIIA filament depicted in this diagram is ∼200 nm long, but our data and other studies show that myosin filaments can be up to 450 nm long in cells. This would double the length scale over which one NMIIA filament could generate contractile forces on the membrane. (B) Blebbistatin treatment weakens the association between NMIIA motor domains and F-actin, causing NMIIA filaments to partially or completely dissociate from the spectrin–F-actin network. This dissociation would lead to a relaxation of the contractile forces on the network, reducing membrane tension and increasing deformability. This relaxation leads to an elongation of RBC shape, as shown in the top view, and a decrease of biconcavity, as shown in the side view (red RBCs). As indicated in the legend below the figure, the F-actin barbed ends are capped by adducin (yellow) and F-actin pointed ends are capped by tropomodulin (dark gray), with tropomyosin rods (purple) spanning their length and protein 4.1R (tan) at the spectrin–F-actin interaction sites (5). Spectrin tetramers are depicted in the folded conformation (40–70 nm) of the native, unspread membrane skeleton, but are able to unfold and extend to nearly 200 nm in length (5, 7).