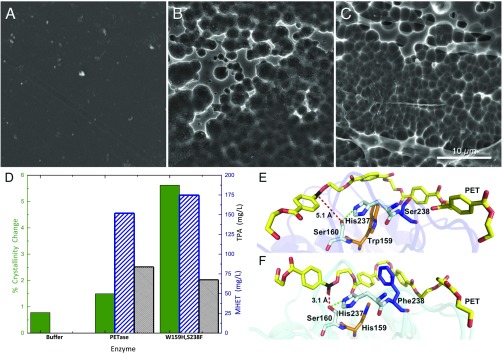
Fig. 3.
Comparison of PETase and the engineered enzyme S238F/W159H with PET. (A) Buffer-only control of PET coupon. (B) PET coupon after incubation with wild-type PETase. (C) PET coupon after incubation with the PETase double mutant, S238F/W159H. All SEM images were taken after 96 h of incubation at a PETase loading of 50 nM (pH 7.2) in phosphate buffer or a buffer-only control. (Scale bar: A–C, 10 μm.) (D) Percent crystallinity change (green, solid bar) and reaction product concentration (MHET, blue diagonal lines; TPA, black hatching) after incubation with buffer, wild-type PETase, and the S238F/W159H-engineered enzymes. (E) Predicted binding conformations of wild-type PETase from docking simulations demonstrate that PET is accommodated in an optimum position for the interaction of the carbon (black) with the nucleophilic hydroxyl group of Ser160, at a distance of 5.1 Å (red dash). His237 is positioned within 3.9 Å of the Ser160 hydroxyl (green dash). Residues Trp159 and Ser238 line the active-site channel (orange and blue, respectively). (F) Double mutant S238F/W159H adopts a more productive interaction with PET. The S238 mutation provides new π-stacking and hydrophobic interactions to adjacent terephthalate moieties, while the conversion to His159 from the bulkier Trp allows the PET polymer to sit deeper within the active-site channel. Two aromatic interactions of interest between PET and Phe238 are at optimal distance (each at 5.4 Å).