Significance
Activated androgen receptor signaling is a key driver to circumvent androgen-deprivation therapy in advanced prostate cancer. Cordon-bleu (COBL) has been identified as a specific player in morphogenesis by regulating actin networks in neurons. Here, we identify another COBL protein, COBL-like 1 (COBLL1), as an important factor that contributes to prostate cancer progression by stimulating androgen receptor signaling and modulating cell morphology. We show that COBLL1 is regulated by androgen and is highly up-regulated in treatment-resistant prostate cancer model cells, where COBLL1 mediates cell proliferation and migration, supporting a fundamental role for COBLL1 in prostate cancer. Our study shows the potential implications for therapeutic targeting of COBLL1 in advanced prostate cancer.
Keywords: prostate cancer, androgen receptor, COBLL1, CDK1, cell morphology
Abstract
Androgen receptor (AR) signaling is essential for prostate cancer progression and acquiring resistance to hormone therapy. However, the molecular pathogenesis through AR activation has not been fully understood. We performed integrative transcriptomic analysis to compare the AR program in a castration-resistant prostate cancer (CRPC) model with that in their parental hormone-sensitive cells. We found that the gene cordon-bleu–like 1 (COBLL1) is highly induced by AR in CRPC model cells. The expression of COBLL1 that possesses an actin-binding domain is up-regulated in clinical prostate cancer tissues and is associated with a poor prognosis for prostate cancer patients. COBLL1 is involved in the cancer cell morphogenesis to a neuron-like cell shape observed in the CRPC model cells, promoting cell growth and migration. Moreover, nuclear COBLL1 interacts with AR to enhance complex formation with CDK1 and facilitates AR phosphorylation for genomic binding in CRPC model cells. Thus, our findings showed the mechanistic relevance of cordon-bleu proteins during the AR-mediated progression to CRPC.
Prostate cancer is the most commonly diagnosed cancer in men worldwide (1). Metastatic prostate cancer is treated by androgen-deprivation therapy because androgen signaling is essential to prostate tumor growth and antiapoptotic ability (2). However, resistance develops quickly during the clinical course and leads to castration-resistant prostate cancer (CRPC) (3). Androgen receptor (AR) functions by interacting with other tissue-specific transcription factors such as forkhead box protein A1 (FOXA1) and modulates epigenetic conditions by recruiting epigenetic factors in the nucleus (4–6). Enhanced AR signaling in CRPC has been observed in several studies (7, 8). ChIP and ChIP-sequencing (ChIP-seq) studies have revealed that the locations of AR-binding sites (ARBSs) are altered in CRPC by differential regulation of AR collaborative factors such as FOXA1 (8–10). Ubiquitin-conjugating enzyme E2 C was identified as a CRPC-specific AR target gene for promoting cell division (8). However, the underlying mechanism promoting AR activation, cell migration, and neuroendocrine transformation of prostate cancer in CRPC remains unclear.
Actin filaments are responsible for nearly all types of cellular movement (11, 12). The cordon-bleu (COBL) family is involved in morphogenetic and patterning processes (13–15). In previous studies, the number of axonal and dendritic branches was increased by overexpression of COBL, and depletion of COBL decreased the number and extent of neurite branching (14, 16). Thus, COBL plays a specific role in morphogenesis. Its N-terminal COBL domain contains three repeated lysine-, arginine-, and proline-rich regions, known as the “KKRAP motif” (16). Although the COBL/KKRAP domain has a ubiquitin-like fold, the precise function of the domain is unknown. In addition, COBL possesses the Wiskott–Aldrich syndrome protein homology 2 (WH2) domain, an actin monomer-binding motif (13, 16, 17). Thus, proteins with WH2 domains confer a flexible array of functions to actin-regulatory proteins.
In the present study, we examined the mechanism of cordon-bleu–like 1 (COBLL1), a paralog of COBL containing a WH2 domain, in cancer progression. We performed genome-wide comparison of ARBSs and gene-expression profiles in AR-positive prostate cancer cells and long-term androgen deprivation (LTAD) cells, which are CRPC model cells. We found that COBLL1, an AR-induced gene, was the most up-regulated gene in LTAD cells compared with parental cells. Our findings suggest critical roles for the cordon-bleu family in prostate cancer progression.
Results
Gene-Expression Profiles Showed Changes in AR-Regulated Genes During the Progression of Prostate Cancer Acquiring Castration Resistance.
To identify the molecular mechanism of the pathogenesis in the transition to CRPC from hormone-dependent prostate cancer, we conducted several comprehensive studies, RNA-sequencing (RNA-seq), microarray analysis, and ChIP-seq in LTAD cells, which were established from androgen-sensitive LNCaP cells. We previously used LTAD cells as CRPC model cells (18, 19). We obtained ARBSs in both the absence and presence of androgen in LTAD cells, in contrast to LNCaP cells (20). A larger number of ARBSs was obtained at a low concentration of dihydrotestosterone (DHT), and enhanced binding signals were observed in LTAD cells compared with LNCaP cells, suggesting the hypersensitivity of AR in CRPC model cells (Fig. S1 A and B).
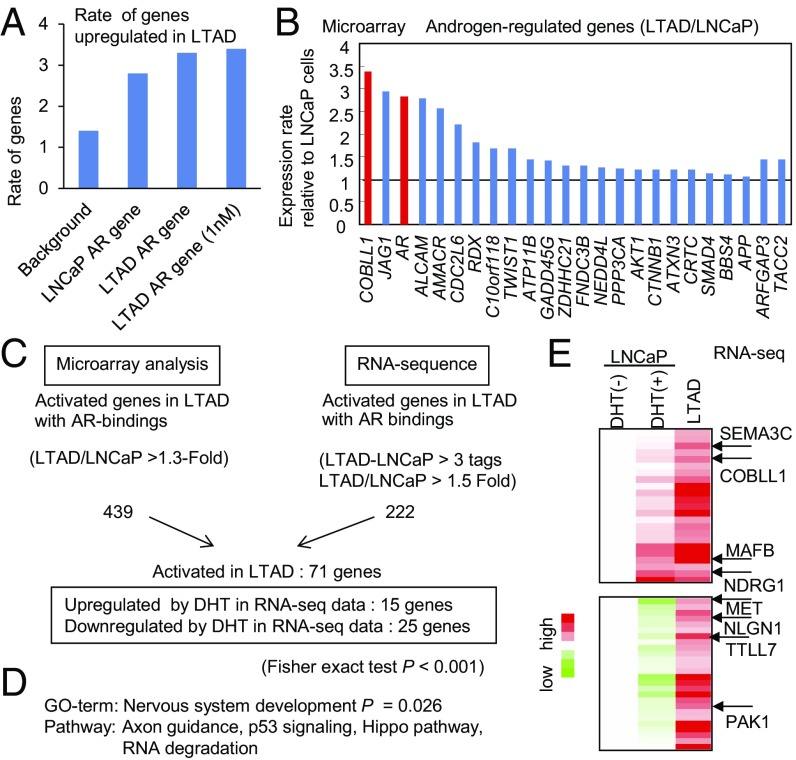
Next, we identified genes up-regulated in the absence of androgen in LTAD cells compared with LNCaP cells by microarray analysis. AR-binding genes (which are located closest to ARBSs in LNCaP cells) such as JAG1 (21) were significantly enriched among these up-regulated genes, suggesting that these AR target genes are involved in cancer progression (Fig. 1A). Among AR-induced genes identified by global analysis in LNCaP cells, such as ARFGAP3 and TACC2 (22, 23), we found that COBLL1 is the gene most up-regulated in the absence of androgen in LTAD cells compared with LNCaP cells (Fig. 1B). Furthermore, to evaluate the results of microarray analysis, we performed RNA-seq analysis in LTAD and LNCaP cells. As observed in the microarray analysis, AR-binding genes were significantly enriched among androgen-induced and up-regulated genes in LTAD cells (Fig. S1 C and D). We then validated a subset of up-regulated genes (71 genes in total) in LTAD cells with AR-binding sites (Fig. 1C). Interestingly, 25 genes were significantly repressed by DHT, suggesting that androgen depletion up-regulates these AR-repressed genes. However, 15 genes (e.g., NDRG1 and JAG1) were significantly induced by androgens, indicating that AR hypersensitivity to a low concentration of androgen also activates the expression of AR-induced genes in CRPC cells. Importantly, gene ontology (GO) term and pathway analysis of these up-regulated genes revealed significant enrichment of neuron development-associated genes (Fig. 1D), such as MAFB (24), MET (25), and SEMA3C (26), suggesting that these neuron-associated genes have important roles in the development of CRPC or the neuroendocrine (NE) phenotype (Fig. 1E). Although the function of COBLL1 is unknown, it may have a function similar to that of COBL, which is an important regulator of actin organization in neurons. Thus, COBLL1 may be an important AR-induced gene in the transition from hormone-sensitive prostate cancer to CRPC cells.
Fig. 1.
Identification of AR-induced genes up-regulated in the absence of androgen in LTAD CRPC model cells by combining microarray, RNA-seq, and ChIP-seq analysis. (A) AR-binding genes are up-regulated significantly in LTAD CRPC model cells. We identified AR-binding genes in LNCaP and LTAD cells in the presence of DHT (10 or 1 nM) by using ChIP-seq data. Up-regulated genes in LTAD cells were found by microarray analysis. (B) COBLL1 is highly up-regulated in LTAD CRPC model cells compared with parental LNCaP cells. By microarray analysis, we analyzed the expression level of AR-induced genes and AR in LTAD cells (without androgen treatment). Fold changes over the expression level in LNCaP cells are shown. (C) Summary of up-regulated AR-binding genes in LTAD CRPC model cells by RNA-seq and microarray analysis. (D) Pathway and GO analysis of AR-binding genes up-regulated in LTAD CRPC model cells. (E) The top 50 genes up-regulated in LTAD CRPC model cells. The results of RNA-seq are summarized in the heat map. Representative genes associated with neuronal function are indicated.
COBLL1 Is Regulated by Androgen and Is Highly Induced in LTAD Cells Compared with LNCaP Cells.
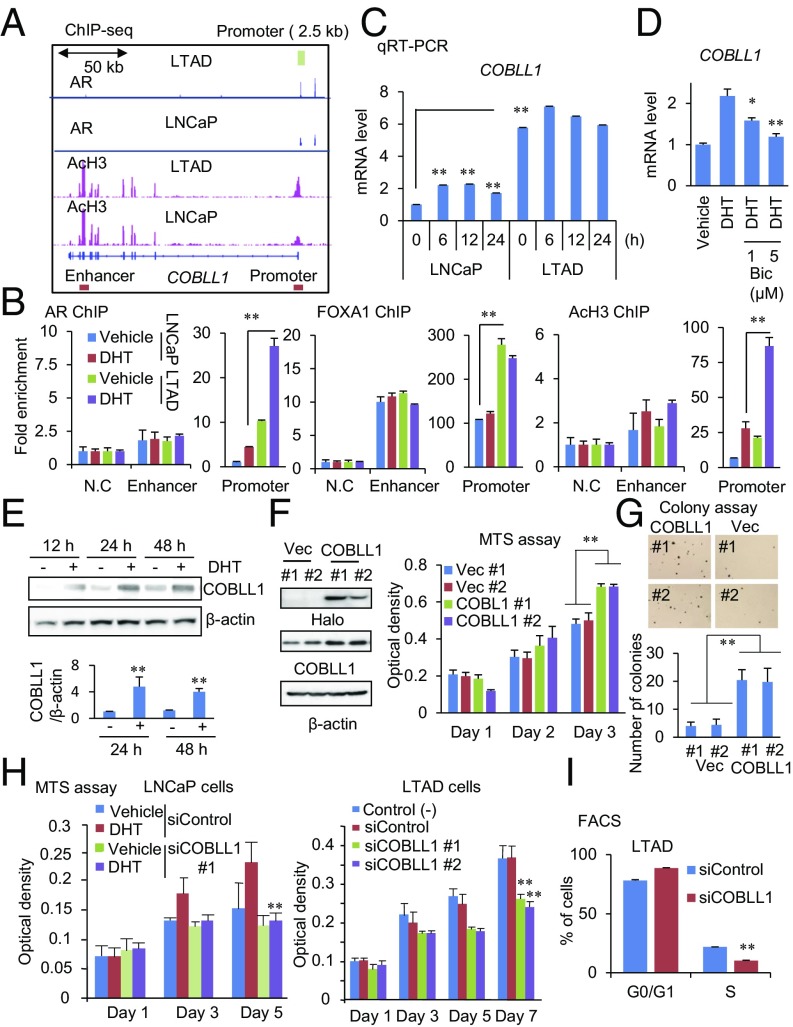
Next, we examined how COBLL1 expression is regulated by the AR transcriptional program. ChIP-seq analysis of AR and AcH3 revealed an ARBS in the COBLL1 promoter region (+817 to +1,127 base pairs relative to the transcriptional start site) which overlapped with the AcH3 peak in DHT-treated LNCaP and LTAD prostate cancer cells (Fig. 2A). We further prepared luciferase reporter constructs with a 2.5-kb region of the wild-type COBLL1 promoter or with mutations in the androgen response element (ARE) site (mutARE) (Fig. S2A). A reporter assay in LNCaP cells showed that DHT treatment markedly enhanced the luciferase activity of the COBLL1 promoter, while this enhancement was impaired in the mutARE construct (Fig. S2B). ChIP-seq and ChIP analysis revealed increased and hypersensitive recruitment of AR as well as histone acetylation in the COBLL1 promoter in LTAD cells compared with LNCaP cells, as well as more enriched FOXA1 binding (Fig. 2 A and B). We also performed an EMSA using COBLL1 ARE and mutARE oligonucleotides. This assay showed that AR binds to the COBLL1 ARE sequence and not to the mutARE sequence (Fig. S2C). Taken together, these results demonstrate that COBLL1 is a putative direct target of AR.
Fig. 2.
AR-regulated COBLL1 promotes CRPC cell growth. (A) Location of AR-binding sites in the COBLL1 locus. ChIP-seq analyses for AR and AcH3 were performed in LNCaP and LTAD cells. AR-binding promoter and enhancer regions (for ChIP) are shown by boxes. The AR promoter 2.5-kb sequence for luciferase assay is shown by a green box. (B) Enhancement of AR, FOXA1, and AcH3 in the AR-binding promoter region of COBLL1 in LTAD cells. Both LNCaP and LTAD cells were treated with 10 nM DHT or vehicle for 24 h. ChIP assays for AR, FOXA1, and AcH3 were performed. Fold enrichments of enhancer and promoter regions were quantified by qPCR (n = 3). N.C., negative control locus. (C) qRT-PCR analysis (n = 3) was performed to determine the expression level of COBLL1 in LNCaP and LTAD cells. Cells were treated with 10 nM DHT for the indicated time. (D) Androgen-mediated induction of COBLL1 is repressed by 1 or 5 μM bicalutamide (Bic). (E) Western blot analysis was performed to examine COBLL1 expression at the protein level in LNCaP cells treated with vehicle or 10 nM DHT. The intensities of COBLL1 bands relative to that of the corresponding β-actin band are shown (n = 3). (F, Left) LNCaP cells stably expressing Halo-COBLL1 or vector control were generated. (Right) The MTS assay was performed to analyze cell growth (n = 4). (G) LNCaP cells overexpressing COBLL1 or vector control (Vec) were plated in plates covered with soft agar. After 3-wk incubation, we counted the number of colonies per field (n = 5). (H) The MTS assay was performed in LNCaP (Left) and LTAD (Right) cells treated with siControl or siCOBLL1 #1 or #2. LNCaP cells were treated with 10 nM DHT or vehicle (n = 4). (I) FACS analysis was performed in LTAD cells treated with siControl or siCOBLL1 #1 for 72 h (n = 3). Values represent the mean ± SD. *P < 0.05; **P < 0.01.
We next investigated androgen-dependent transcriptional regulation of COBLL1 expression. RNA-seq and qPCR analysis demonstrated that COBLL1 mRNA was significantly induced (by ∼2.5-fold) at 6 h after DHT treatment relative to the vehicle control LNCaP cells (Fig. 2C and Fig. S2D). Notably, marked up-regulation of COBLL1 expression in LTAD cells was observed by qPCR analysis. Similar results were observed in VCaP cells (Fig. S2 E and F). Treatment with bicalutamide or short interference (si) AR blocked the induction by DHT treatment or enhanced the expression of COBLL1 in LTAD cells (Fig. 2D and Fig. S2 G–K). We also observed induction of COBLL1 protein by androgen in LNCaP and VCaP prostate cancer cells (Fig. 2E and Fig. S2L). Although COBLL1 was highly expressed in VCaP cells compared with LNCaP cells, COBLL1 expression was higher in AR-positive prostate cancer cells, supporting the importance of AR in inducing this gene (Fig. S2 M and N).
COBLL1, Associated with F-Actin, Sustains the Neuron-Like Cell Shape of CRPC Model Cells.
We then examined the involvement of COBLL1 in prostate cancer aggressiveness and castration resistance. To analyze the endogenous function of COBLL1, we established two clones of LNCaP cells stably overexpressing COBLL1 (Fig. 2F). Overexpression of COBLL1 accelerated prostate cancer cell proliferation and anchorage-independent cell growth (Fig. 2 F and G). Moreover, two siRNAs targeting COBLL1 efficiently knocked down COBLL1 by Western blot analysis (Fig. S3 A and B). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay revealed that knockdown of COBLL1 impaired androgen-dependent cell growth of LNCaP, VCaP, and castration-resistant LTAD and 22Rv1 cells (Fig. 2H and Fig. S3 C and D). Cell migration and cell-cycle progression of LTAD cells were also inhibited by COBLL1 knockdown (Fig. 2I and Fig. S3 E–G).
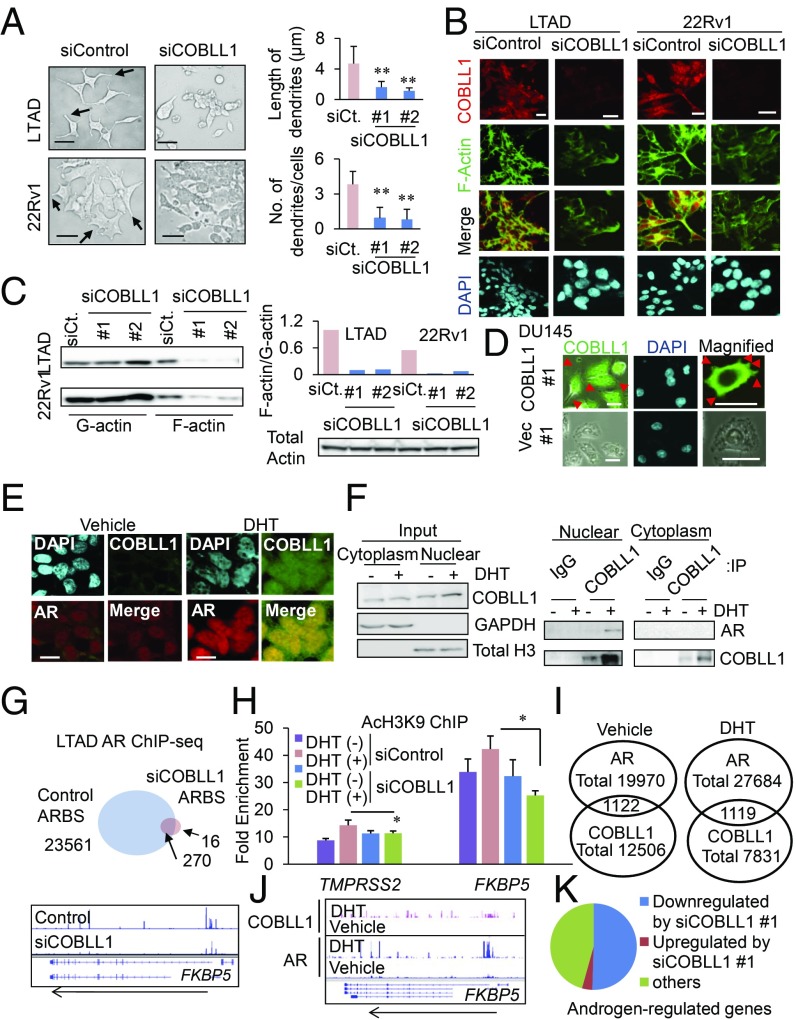
Furthermore, we analyzed the roles of COBLL1 in regulating CRPC cell morphology and actin organization, as COBLL1 has one actin-binding WH2 domain. LTAD cells as well as 22Rv1 cells showed morphological changes toward a neuron-like phenotype with multiple extensions (Fig. 3A). The cells exhibited a rounded epithelial phenotype following COBLL1 knockdown. Quantitative analysis revealed that both the number and length of dendrites were repressed, suggesting that COBLL1 plays an important role in these cell-shape changes in CRPC model cells (Fig. 3A). Analysis by phalloidin staining, which reflects the F-actin distribution, revealed dense actin bundles in the peripheral regions of these cells and colocalization of actin with COBLL1 (Fig. 3B). This dense actin staining and organization in cells was repressed by COBLL1 knockdown (Fig. 3B). The amount of F-actin relative to G-actin was decreased in COBLL1-knockdown cells, indicating that COBLL1 affects the dynamics of actin organization in prostate cancer (Fig. 3C). We next established AR-negative DU145 cells stably overexpressing COBLL1. COBLL1 overexpression induced the growth of DU145 cells (Fig. S3H). Interestingly, overexpression of COBLL1 in DU145 cells induced microvilli-like dendrites on the cell surface (Fig. 3D). We also found that COBLL1 overexpression promoted cell migration and soft agar colony formation in DU145 and RWPE cells (Fig. S3 I and J). Furthermore, we observed decreased cell growth and migration by knockdown of COBLL1 in PC3 cells, in which the COBLL1 expression level was higher than in other AR-negative cells (Figs. S2N and S3 K–M). Taken together, our data strongly support the notion that COBLL1 plays an important role in the change of cells to a neuron-like shape by regulating actin organization.
Fig. 3.
COBLL1 regulates actin organization and AR genomic bindings. (A, Left) Dendrite-like structures from CRPC model cells were diminished by COBLL1 knockdown. Microscopic images of LTAD and 22Rv1 cells are shown. Dendrite-like structures from CRPC model cells are indicated by arrows. (Scale bars: 10 μm.) (Right) Cells were treated with siControl or siCOBLL1 #1 or #2, and the length of dendrites and the number of dendrites in LTAD cells were determined (n = 16–20). (B) The organization of actin filaments was inhibited by COBLL1 knockdown. Both LTAD and 22Rv1 cells were treated with siControl or siCOBLL1 #1 for 72 h. Immunofluorescence analysis using anti-COBLL1 antibody and phalloidin was performed. COBLL1 colocalization with F-actin was observed in the dense actin bundles in the cytoplasm. (Scale bars: 10 μm.) (C, Left) F-actin and G-actin in LTAD and 22Rv1 cells treated with siControl or siCOBLL1 #1 or #2 were fractioned and subjected to Western blot analysis with β-actin antibody. (Right) The ratio of F-actin to G-actin band intensities was determined. (D) COBLL1 overexpression changed the morphology of prostate cancer cells. Immunofluorescence analysis was performed in DU145 cells overexpressing COBLL1 or vector control. Increased dendrite-like structures in DU145 cells are indicated by arrowheads. (E) LNCaP cells were treated with 10 nM DHT or vehicle for 24 h. Immunofluorescence analysis was performed to analyze the expression of AR and COBLL1 proteins. (Scale bars: 10 μM.) (F) COBLL1 associates with AR in the nucleus. Immunoprecipitation (IP) with normal IgG or COBLL1 antibody and Western blot analysis with AR, COBLL1, GAPDH, and total H3 antibodies was performed in LNCaP cells. Cells were treated with 10 nM DHT or vehicle for 24 h. (G, Upper) The number of AR-binding sites was severely decreased by COBLL1 knockdown. LTAD cells were treated with siControl or siCOBLL1 #1 for 48 h and then were treated with 10 nM DHT for 24 h. The Venn diagram shows the number of AR-binding sites in LTAD cells found by AR ChIP-seq analysis. (Lower) A representative locus of the AR-regulated gene FKBP5 is shown. (H) Androgen-mediated histone modification of AR-binding sites was impaired by COBLL1 knockdown. LTAD cells were treated with siControl or siCOBLL1 #1 for 48 h and then were treated with 10 nM DHT or vehicle for 24 h. A ChIP assay was performed using anti-AcH3 antibody. Fold enrichment over input was measured by qPCR (n = 3). (I) Overlap of COBLL1-binding sites with AR. The numbers of significant AR- (P value < 10E-5) or COBLL1- (P value < 10E-4) binding sites identified by model-based analysis of ChIP-seq (MACS) are shown. (J) Tag distributions of COBLL1 ChIP-seq are shown around the FKBP5 locus. (K) We treated LTAD cells with siControl or siCOBLL1 #1 for 48 h and then with 10 nM DHT or vehicle for 24 h. Gene regulation by androgen was analyzed by RNA-seq. Genes induced by androgen (>twofold) were selected. Values represent the mean ± SD. *P < 0.05; **P < 0.01.
COBLL1 Promotes AR-Mediated Gene Inductions and Histone Activation.
Notably, COBLL1 was also expressed in the nucleus according to immunofluorescence (Fig. 3E) and Western blot (Fig. 3F) analyses. We observed androgen-dependent nuclear enrichment of AR colocalized with COBLL1 in LNCaP cells. In addition, we observed that AR interacted with COBLL1 based on immunoprecipitation and Western blot analyses (Fig. 3F). Furthermore, we determined the interaction region of COBLL1 with AR. In immunoprecipitation analysis, we found that the WH2 domain of COBLL1 and the N-terminal, activation function 1 (AF1) domain of AR are important for the interaction of COBLL1 with AR (Fig. S4 A–D). Moreover, the interaction of COBLL1 with AR was also observed in VCaP cells (Fig. S4 E and F).
We then examined by ChIP-seq whether COBLL1 modulates AR signaling in CRPC model cells. Loss of COBLL1 dramatically blocked genomic binding of AR in LTAD cells (Fig. 3G). This inhibition of AR binding is associated with histone modifications in AR-binding enhancer regions (Fig. 3H). Furthermore, we identified global COBLL1-binding regions by ChIP-seq and found significant overlap of COBLL1 with ARBSs (Fig. 3 I and J). Motif analysis showed that FOXA1- and AR-binding motifs were enriched in COBLL1-binding peaks, particularly in the presence of DHT (Fig. S5A). Consistent with the role of COBLL1 in AR-binding ability, we found significant enrichment of androgen-regulated genes among COBLL1-binding genes in the absence and presence of DHT (Fig. S5B). Moreover, COBLL1-binding sites in the presence of DHT were distributed mainly around the promoter regions of target genes, indicating the role of COBLL1 in the transactivation of these gene expressions (Fig. S5C). Next, by RNA-seq analysis, we found that COBLL1 knockdown impaired androgen-mediated gene induction in LTAD cells (Fig. 3K). In contrast, COBLL1 overexpression induced AR binding, histone H3 acetylation, and androgen-regulated gene expression (Fig. S5D). Positive regulation of AR-mediated transcription was also observed in VCaP cells (Fig. S5E). Taken together, these results reveal that COBLL1 activates AR downstream signals by enhancing AR binding in the nucleus of CRPC cells.
AR Phosphorylation (Ser81) Is Driven by COBLL1 Expression.
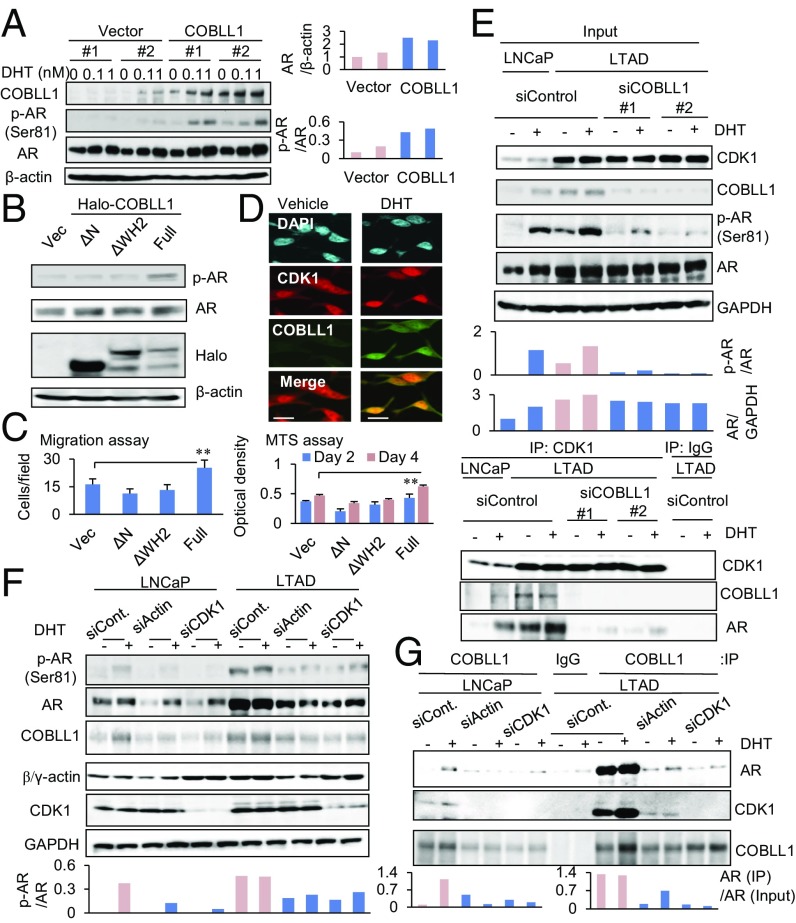
Furthermore, we investigated the mechanism of COBLL1 in activating AR signaling. Interestingly, we observed that COBLL1 overexpression in LNCaP cells promoted androgen-dependent AR phosphorylation and protein level at a low concentration of DHT (Fig. 4A). However, the COBLL1 deletion mutants ΔN and ΔWH2 failed to enhance the AR phosphorylation (Fig. 4B), cell proliferation, and migration of LNCaP cells (Fig. 4C), suggesting that full-length COBLL1 is necessary for this activation. Ser81 is the site of posttranslational modification of AR by androgen treatment. Cyclin-dependent kinase 1 (CDK1) is responsible for this posttranslational modification and AR protein stability (27–29), and we demonstrated that CDK1 interacts with AR in the nucleus (Fig. S6A). Moreover, immunofluorescence analysis revealed an interaction between COBLL1 and CDK1 in the nucleus (Fig. 4D). We also observed an interaction in VCaP and 22Rv1 CRPC model cells (Fig. S6B). Furthermore, in accordance with the up-regulation of CDK1, enhanced interaction of CDK1 with COBLL1 and AR was observed in LTAD cells (Fig. 4E). COBLL1 knockdown severely blocked AR phosphorylation (Ser81) and protein level in LTAD cells (Fig. 4E). Notably, we observed that the CDK1 interaction with AR was inhibited by COBLL1 knockdown in LTAD cells, although CDK1 was highly expressed in LTAD cells (Fig. 4E). Consistent with the decreased phosphorylation of AR, we found that nuclear enrichment of AR was impaired by COBLL1 knockdown (Fig. S6C). These results suggest the potential role of COBLL1 as a key factor in the CDK1 association with AR in CRPC cells.
Fig. 4.
COBLL1 enhances AR phosphorylation (Ser81) via interacting with CDK1. (A, Left) COBLL1 overexpression accelerates AR phosphorylation. LNCaP cells overexpressing COBLL1 or vector controls were treated with vehicle or with 0.1 or 1 nM DHT for 24 h. Western blot analysis was performed to detect the indicated proteins. (Right) The ratios of p-AR band intensity relative to AR and of AR band intensity relative to β-actin are shown. (B) COBLL1 mutants failed to activate AR. LNCaP cells were transfected with empty vector, Halo-COBLL1, or its ΔN or ΔWH2 mutants. After 48-h incubation, cells were treated with 1 nM DHT for 24 h, and Western blot analysis was performed. (C) Migration (Left) and MTS (Right) assays were performed. Values represent the mean ± SD. **P < 0.01. (D) Colocalization of CDK1 and COBLL1 in the nucleus of LNCaP cells. LNCaP cells were treated with 10 nM DHT or vehicle for 24 h. An immunofluorescence analysis was performed to analyze the expression of CDK1 and COBLL1 proteins. (Scale bars: 10 μm.) (E) COBLL1 is indispensable for the interaction of CDK1 with COBLL1 and AR. LNCaP and LTAD cells were treated with siControl or siCOBLL1 #1 or #2 for 48 h. Then cells were treated with 1 nM DHT or vehicle for 24 h. Immunoprecipitation was performed using anti-CDK1 antibody. (Upper) Western blot analysis was performed to detect the indicated proteins. (Lower) The ratios of p-AR band intensity relative to AR and of AR band intensity relative to GAPDH are shown. (F and G) Loss of actin/CDK1 alleviates the interaction of COBLL1 with AR for AR activation. LNCaP and LTAD cells were treated with siControl, siActin, or siCDK1 for 48 h. Then cells were treated with 10 nM DHT or vehicle for 24 h. Immunoprecipitation was performed using anti-COBLL1 antibody. (F and G, Upper) Western blot analysis was performed to detect the indicated proteins of input samples (F) or immunoprecipitation samples (G). (F and G, Lower) The ratios of p-AR band intensity to AR (F) and of AR band immunoprecipitation samples to input AR (G) are shown.
We further evaluated whether the COBLL1 interaction with AR depends on actin because the actin-binding WH2 domain is important for this interaction (Fig. S4C). Interestingly, knockdown of actin inhibited the association of COBLL1 with AR as well as the phosphorylation and protein level of AR, suggesting the involvement of actin in COBLL1/AR complex formation and AR activity (Fig. 4 F and G). Moreover, we observed that knockdown of CDK1 abolished the interaction of COBLL1 with AR (Fig. 4 F and G). Although the CDK1 interaction with COBLL1 did not depend on the WH2 domain (Fig. S6D), overexpression of CDK1 enhanced the interaction of COBLL1 with AR (Fig. S6E). These results indicate that the CDK1 and actin interactions with COBLL1 promote the recruitment of AR to this complex and subsequent AR activation (Fig. S6F).
Knockdown of COBLL1 Inhibits Castration-Resistant Prostate Tumor Growth in Vivo.
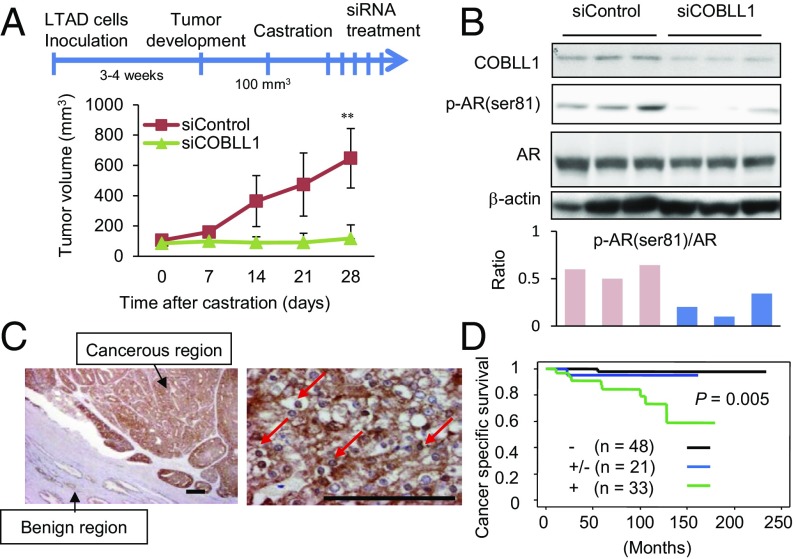
Next, we further examined the role of COBLL1 in castration-resistant tumor growth by knocking down COBLL1 in vivo. First, LTAD cells were used as a model CRPC xenograft and were s.c. implanted into nude mice (Fig. 5A). Interestingly, siCOBLL1 treatment inhibited LTAD tumor growth after castration, indicating the potential involvement of COBLL1 in CRPC tumor growth (Fig. 5A). By Western blot analysis, we observed that COBLL1 protein expression levels were markedly reduced by siCOBLL1 injection (Fig. 5B). Similar results were observed in xenograft assays using another CRPC model, 22Rv1-derived tumors (Fig. S7 A and B).
Fig. 5.
High expression of COBLL1 promotes CRPC tumor growth and correlates with poor prognosis of patients. (A) Nude mice bearing LTAD cell-derived tumors were castrated after tumor development, and tumors were treated with the siControl or siCOBLL1 #1. Tumor volumes at the indicated times are shown (n = 8). Values represent the mean ± SD. **P < 0.01. (B, Upper) Immunoblotting of tumor lysates with the indicated antibodies (n = 3). (Lower) The ratio of p-AR band intensity relative to AR is shown. (C) Representative images of immunohistochemical staining of COBLL1 in prostate cancer sections (n = 102). Arrows indicate nuclear enrichment of COBLL1 protein. (Scale bars: 50 µm.) (D) Cancer-specific survival rates of patients determined by the Kaplan–Meier method. The P value was obtained by log-rank test.
Next, we analyzed the pathophysiological significance of COBLL1 expression in human prostate cancer tissues by immunohistochemical analysis (Fig. 5C). We observed increased expression of COBLL1 protein in both the cytoplasmic and nuclear regions in prostate cancer tissues. Immunohistochemistry results revealed that high expression of COBLL1 was significantly correlated with a poor prognosis (Fig. 5D) and with high Gleason scores (Fig. S7C) in prostate cancer patients. According to publicly available microarray datasets of prostate cancer clinical samples from GEO (https://www.ncbi.nlm.nih.gov/geo/) or The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov), COBLL1 and COBL mRNA expression was significantly elevated in prostate cancer samples compared with benign samples (Fig. S7 D and E). Importantly, multivariate analysis showed that high expression of COBLL1 is an independent prognostic factor in prostate cancer patients (Fig. S7F). These results suggest that both nuclear and cytoplasmic expression of COBLL1 is important for prostate cancer progression.
Finally, because neuron-associated genes were enriched among AR-binding genes in CRPC model cells, and neuron-like cell-morphology changes were observed, we further investigated whether COBLL1 promotes NE cell phenotypes by analyzing the expression of the canonical genes synaptophysin (SYP), chromogranin A (CHGA), and neuron-specific γ-enolase (NSE). LTAD cells showed increased expression of these NE markers compared with parental cells (Fig. S8A). Interestingly, we found an association between COBLL1 and SYP at the protein level (Fig. S8B). Moreover, we demonstrated that these expression changes were inhibited by COBLL1 knockdown (Fig. S8 C and D). In contrast, CHGA and NSE expression was induced in COBLL1-overexpressing cells, suggesting the involvement of COBLL1 in the progression to NE-like prostate cancer (Fig. S8E). Our COBLL1 ChIP-seq data indicate that genomic functions of COBLL1 are involved in such gene-expression changes (Fig. S8F).
Discussion
Our study identified that COBLL1 acts as a key player in cancer progression by activating the invasive and proliferative phenotype of prostate cancer cells. The biological relevance of COBL has been documented mainly in neuron development by promoting axon protrusion (14, 15, 30–32). In contrast, the molecular function and regulatory mechanism of COBLL1 have not been thoroughly investigated. The COBLL1 locus is genetically associated with the development of metabolic diseases (33) and cortical surface (34). High levels of COBLL1 expression are clinically associated with leukemia (35, 36). COBLL1 may be involved in the NF-κB signaling in leukemia cells by stabilizing IKKγ (35), although the molecular functions of COBLL1 are unclear. We found that COBLL1 is transcriptionally regulated by androgen. Expression of COBLL1 was highly up-regulated in the absence of androgen in CRPC model cells. This result indicates that COBLL1 functions as an AR downstream molecule in prostate cancer progression. In our global analysis of gene-expression profiles, a subset of genes induced by AR was up-regulated in LTAD cells compared with LNCaP cells, suggesting that the hypersensitive AR activity observed in LTAD cells is correlated with such induction of AR-binding genes. As another possibility for inducing AR-binding genes in CRPC cells, previous reports (8–10) have documented the selection and reprogramming of AR downstream molecules correlated with AR collaborators such as FOXA1 or cofactor distributions.
The cordon-bleu proteins COBL and COBLL1 are known to possess KKRAP motifs and WH2 domains (16, 17). We found that COBL is not induced by androgen in LNCaP cells but instead is repressed in LTAD cells compared with LNCaP cells. The expression level of COBL is very low compared with COBLL1 (SI Results and Fig. S9). Therefore, these results indicate that COBLL1 is the dominant protein among members of the COBL family for modulating actin assembly in prostate cancer. We first evaluated whether COBLL1 promotes prostate cancer cytoskeletal organization by modulating actin remodeling while enhancing cell growth and migration. We also observed long dendrites from CRPC model cells, which changed the cancer cells toward a neuron-like cell shape. Inhibition of COBLL1 reduced F-actin staining and axon-like extensions, suggesting a role of COBLL1 in this actin assembly for CRPC growth. Next, we found that the expression of NE markers such as SYP and CHGA (37) was increased in LTAD cells and was regulated by COBLL1 at the mRNA level. Nuclear COBLL1 may be involved in such gene-expression changes, although further analyses of the mechanism will be required. In agreement with our results, previous studies showed that F-actin–rich ruffles are generated at the periphery of COS7 cells by overexpressing COBL (14). Taken together, these findings suggest that COBLL1 plays an important role in NE-like cell morphogenesis during prostate cancer progression.
Another interesting characteristic of COBLL1 is the nuclear function of COBLL1 protein in prostate cancer cells. Our global analysis of AR- and COBLL1-associated regions demonstrated the important role of COBLL1 in AR genomic binding. As a molecular mechanism, we revealed that COBLL1 functions by promoting AR phosphorylation (Ser81) for AR activation. CDK1 is a key molecule that enhances the phosphorylation and stability of AR in CRPC (27–29). We found that the interaction between CDK1 and COBLL1 was enhanced in LTAD cells. Importantly, COBLL1 knockdown reduced the AR interaction with CDK1, suggesting the involvement of COBLL1 in this step. Moreover, our immunoprecipitation analysis indicates the importance of the actin-binding WH2 domain of COBLL1, actin, and CDK1 in the formation of the COBLL1/CDK1/AR complex in the nucleus. Thus, we present a working model in which COBLL1 is an adaptor protein used to assemble the complex of actin, CDK1, and AR to promote the phosphorylation step for genomic binding in CRPC.
In summary, we found that AR-targeted COBBL1 is involved in the progression of CRPC. Through its actin-binding ability, COBLL1 is a potent growth and migration promoter that modulates actin organization in cancer cells. This unique feature as an activator of AR genomic binding makes COBLL1 a potential specific therapeutic target for CRPC.
Materials and Methods
ChIP-Seq.
ChIP was performed as described previously (18–20), using anti-AR and anti-COBLL1 antibodies. ChIP-seq analyses were performed using a HiSeq platform (Illumina).
RNA-Seq.
RNA-seq library construction and sequencing were performed according to the manufacturer’s protocol for the SOLiD 4 System (Applied Biosystems).
Xenograft Model.
Prostate cancer LTAD cells in medium were mixed with Matrigel (BD Biosciences) and s.c. injected into one side of 5-wk-old male BALB/C nude mice (Nihon Crea). After tumor development, we performed castration; then siCOBLL1 or siControl complexed with Opti-MEM and Lipofectamine RNAiMAX (Thermo Fisher Scientific) was injected into tumors. All animal experimental protocols were performed in accordance with Tokyo Metropolitan Institute of Gerontology animal experiment guidelines. The ethics committee of animal experiments at the Tokyo Metropolitan Institute of Gerontology approved the study protocol.
Clinical Samples.
The University of Tokyo ethics committee approved our study (G10044-2), and informed consent was obtained from each patient before surgery. We obtained 102 prostate cancer samples from surgeries performed at the University of Tokyo Hospital. Detailed materials and methods regarding xenograft model, qRT-PCR, immunoprecipitation and Western blot analysis, cell growth and migration assays, microarray analysis, F-actin/G-actin ratio measurement, siRNA transfection, and immunohistochemistry, immunofluorescence, and statistical analyses are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank RIKEN for sequencing our samples. This work was supported by grants from the Cell Innovation Program, the Project for Development of Innovative Research on Cancer Therapeutics and the Project for Cancer Research and Therapeutic Evolution (Grant JP17cm0106117) of the Ministry of Education, Culture, Sports, Science and Technology, and the Japan Agency for Medical Research and Development (to S.I.); by Japan Society for the Promotion of Science Grants 15K15581, 15K15353, and 17H04334 (to K.T. and S.I.); by the Program for Promotion of Fundamental Studies in Health Sciences, National Institute of Biomedical Innovation (S.I.); by grants from the Terumo Foundation for Life Sciences and Arts, Japan (to K.T.); by grants from the NOVARTIS Foundation (Japan) for the Promotion of Science (to K.T.); by grants from Takeda Science Foundation, Japan (to S.I. and K.T.); and by Uehara Memorial Foundation, Japan Grant 201520122j (to S.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession nos. GSE94028, GSE94577, and GSE100239).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721957115/-/DCSupplemental.
References
- 1.Balk SP. Androgen receptor functions in prostate cancer development and progression. Asian J Androl. 2014;16:561–564. doi: 10.4103/1008-682X.126396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, et al. Defining a population of stem-like human prostate cancer cells that can generate and propagate castration-resistant prostate cancer. Clin Cancer Res. 2016;22:4505–4516. doi: 10.1158/1078-0432.CCR-15-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, et al. A role for WDR5 in integrating threonine 11 phosphorylation to lysine 4 methylation on histone H3 during androgen signaling and in prostate cancer. Mol Cell. 2014;54:613–625. doi: 10.1016/j.molcel.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahu B, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin HJ, Zhao JC, Wu L, Kim J, Yu J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat Commun. 2014;5:3972. doi: 10.1038/ncomms4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez R. Actin filament nucleation and elongation factors–Structure-function relationships. Crit Rev Biochem Mol Biol. 2009;44:351–366. doi: 10.3109/10409230903277340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesarone MA, Goode BL. Actin nucleation and elongation factors: Mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanelli AM, Klingensmith J. The actin nucleator cordon-bleu is required for development of motile cilia in zebrafish. Dev Biol. 2011;350:101–111. doi: 10.1016/j.ydbio.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja R, et al. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007;131:337–350. doi: 10.1016/j.cell.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwintzer L, et al. The functions of the actin nucleator Cobl in cellular morphogenesis critically depend on syndapin I. EMBO J. 2011;30:3147–3159. doi: 10.1038/emboj.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renault L, Bugyi B, Carlier MF. Spire and cordon-bleu: Multifunctional regulators of actin dynamics. Trends Cell Biol. 2008;18:494–504. doi: 10.1016/j.tcb.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Wayt J, Bretscher A. Cordon bleu serves as a platform at the basal region of microvilli, where it regulates microvillar length through its WH2 domains. Mol Biol Cell. 2014;25:2817–2827. doi: 10.1091/mbc.E14-06-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayama K, et al. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32:1665–1680. doi: 10.1038/emboj.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama K, et al. TET2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression. Nat Commun. 2015;6:8219. doi: 10.1038/ncomms9219. [DOI] [PubMed] [Google Scholar]
- 20.Takayama K, et al. Dysregulation of spliceosome genes by RNA-binding protein PSF in advanced prostate cancer. Proc Natl Acad Sci USA. 2017;114:10461–10466. doi: 10.1073/pnas.1706076114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santagata S, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–6857. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 22.Takayama K, et al. Integration of cap analysis of gene expression and chromatin immunoprecipitation analysis on array reveals genome-wide androgen receptor signaling in prostate cancer cells. Oncogene. 2011;30:619–630. doi: 10.1038/onc.2010.436. [DOI] [PubMed] [Google Scholar]
- 23.Takayama K, Inoue S. Transcriptional network of androgen receptor in prostate cancer progression. Int J Urol. 2013;20:756–768. doi: 10.1111/iju.12146. [DOI] [PubMed] [Google Scholar]
- 24.Blanchi B, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 25.Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyas I, Bozon M, Moret F, Castellani V. Motoneuronal Sema3C is essential for setting stereotyped motor tract positioning in limb-derived chemotropic semaphorins. Development. 2012;139:3633–3643. doi: 10.1242/dev.080051. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci USA. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Gulla S, Cai C, Balk SP. Androgen receptor serine 81 phosphorylation mediates chromatin binding and transcriptional activation. J Biol Chem. 2012;287:8571–8583. doi: 10.1074/jbc.M111.325290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. Positive feedback loop mediated by protein phosphatase 1α mobilization of P-TEFb and basal CDK1 drives androgen receptor in prostate cancer. Nucleic Acids Res. 2017;45:3738–3751. doi: 10.1093/nar/gkw1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortune MD, et al. Statistical colocalization of genetic risk variants for related autoimmune diseases in the context of common controls. Nat Genet. 2015;47:839–846. doi: 10.1038/ng.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mez J, et al. Alzheimer’s Disease Genetics Consortium Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 2017;13:119–129. doi: 10.1016/j.jalz.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou W, et al. The actin nucleator Cobl is controlled by calcium and calmodulin. PLoS Biol. 2015;13:e1002233. doi: 10.1371/journal.pbio.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai DC, et al. A genome-wide search for quantitative trait loci affecting the cortical surface area and thickness of Heschl’s gyrus. Genes Brain Behav. 2014;13:675–685. doi: 10.1111/gbb.12157. [DOI] [PubMed] [Google Scholar]
- 35.Han SH, et al. Cobll1 is linked to drug resistance and blastic transformation in chronic myeloid leukemia. Leukemia. 2017;31:1659. doi: 10.1038/leu.2017.107. [DOI] [PubMed] [Google Scholar]
- 36.Plesingerova H, et al. COBLL1, LPL and ZAP70 expression defines prognostic subgroups of chronic lymphocytic leukemia patients with high accuracy and correlates with IGHV mutational status. Leuk Lymphoma. 2017;58:70–79. doi: 10.1080/10428194.2016.1180690. [DOI] [PubMed] [Google Scholar]
- 37.Mu P, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueyama K, et al. Knockdown of Efp by DNA-modified small interfering RNA inhibits breast cancer cell proliferation and in vivo tumor growth. Cancer Gene Ther. 2010;17:624–632. doi: 10.1038/cgt.2010.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.