Fig. 7.
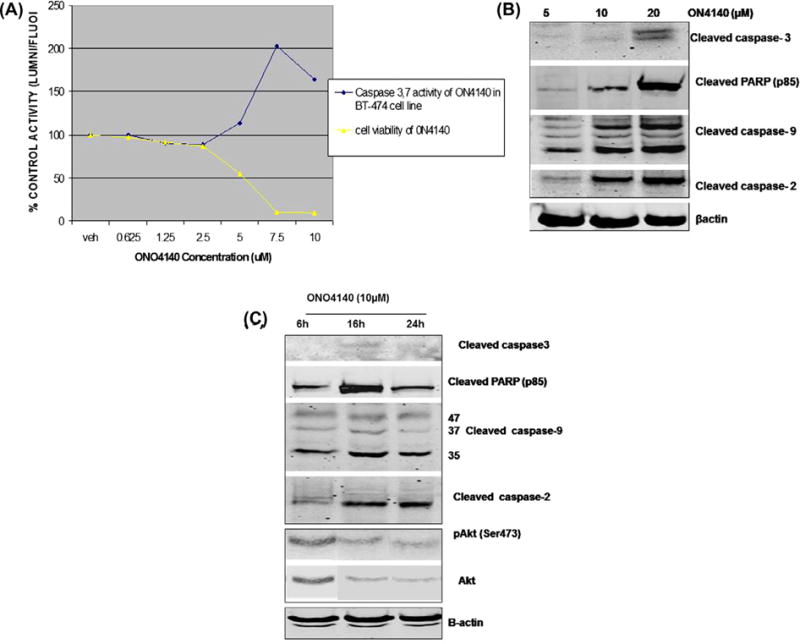
The PI(3)K-Akt pathway and the intrinsic apoptotic pathway are targets of Hsp90 inhibitor-mediated cell death. (A) Caspase 3,7 activity and cell viability of ONO4140 in BT-474 cell line. BT-474 cells were treated with the indicated doses of ONO4140 for 72 h to measure apoptosis by Caspase Glow assay. The assay measures effector Caspase-3,7 activity as the potency of ONO4140 in cleaving the luminogenic Caspase 3/7 substrate containing tetrapeptide sequence-DEVD and generating glow type luminescent signal. The percentage increase in apoptotic cells was calculated by comparing the luminescence readings obtained from ONO4140 treated cells with those obtained from vehicle-treated cells. Caspase-3/7 activity in vehicle-treated cells was normalised to 100%. The increase in Caspase activity from ONO4140 treated cells also corresponds to decrease in cell viability (as measured by Cell Titer-Blue assay described in Section 2.8). (B) Activation of Caspase 9–Caspase 3 intrinsic apoptotic pathway upon exposure to ONO4140. BT-474 cells were treated for 24 h with indicated concentrations of ONO4140 compound and cells were lysed for western blot analysis for various Caspases. (C) The kinetics of Caspase 9–Caspase 3 intrinsic apoptotic pathway upon exposure to ONO4140 (10 μM). BT-474 cells were treated with ONO4140 compound (10 μM) for different time periods and cells were lysed for western blot analysis. β-actin was used as loading control for western blots. Maximum activation of caspase 9, and/degradation of Akt occur at the same time point i.e. 16 h post treatment (at 10 μM), suggesting that inhibition of hsp90 by ONO4140 results in the inhibition of HER2/PI3 K/Akt pathway which in turn results in the activation of Caspase 9–Caspase 3 intrinsic apoptotic pathway.
