Table 3.
Oxidation of (Z) and (E)-1,3,5-trimethoxy-2-[2-(4-methoxy-3-nitrobenzylsulfanyl)vinyl]benzene (11 and 12)
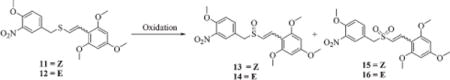
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Compd | Oxidizing agent (eq.) | Solvent | Temp (°C) | Time (h) | Producta | Yieldb (%) |
| 1 | 11 | H2O2 | AcOH | rt | 8 | — | —c |
| 2 | 12 | H2O2 | AcOH | rt | 24 | 16 | 40 |
| 3 | 11 | H2O2 | AcOH | 5 | 6 | 13 | 55 |
| 4 | 12 | H2O2 | AcOH | 5 | 6 | 14 | 75 |
| 5 | 12 | H2O2 | AcOH | 60 | 6 | 16 | 85 |
| 6 | 11 | m-CPBA (2.5) | MeOH | rt | 12 | — | —c |
| 7 | 12 | m-CPBA (2.5) | MeOH | rt | 24 | 16 | 50 |
| 8 | 11 | Oxonee (4) | THF/MeOH (2 : 1) | rt | 24 | 13 & 15d | 55 |
| 9 | 11 | Oxonee (6) | THF/MeOH (2 : 1) | rt | 24 | 15 | 65 |
| 10 | 11 | H2O2 | Hexafluoro-2-propanol | rt | 2 | 13 | 40 |
Starting compound geometry was retained in the product.
Yield of isolated product.
Starting compound decomposed.
Partial oxidation leading to mixture of sulfoxide and sulfone (ratio:15/85).
Oxone was dissolved in water (2 mL g−1) and then added to the reaction mixture.
