Abstract
Physiological heterogeneity among single cells with identical genetic information has been observed in a large number of bacterial phenotypes, including growth, stress responses, cell size, and antibiotic tolerance. Despite the widespread observation of this phenomenon in bacterial populations, not much is known about the molecular mechanisms behind phenotypic heterogeneity. Currently, our understanding is primarily limited to transcriptional profile of single cells using fluorescence reporters. Although the development of these tools has been extremely informative, it cannot fully explain the heterogeneity seen in populations. In a recent publication, Fan et al. have developed a dual-fluorescent reporter system that is capable of quantitatively measuring translational fidelity in single cells. It is shown that translational fidelity is heterogeneous and affects the growth characteristics of single cells. The development of tools for analysis of molecular heterogeneity downstream of transcription may play an important role in advancing our understanding of the physiology of bacterial populations.
Keywords: Protein Synthesis, Genetic Code, Single Cell
Gene expression heterogeneity within bacterial populations is best understood at the transcriptional level. Gene expression was first shown to be stochastic by Elowitz et al. (Elowitz, et al. 2002), where their work shows that two mostly identical copies of reporter genes are expressed at different levels in the same cell, especially when the transcriptional level becomes low. Later work demonstrates that transcription of individual genes is bursty with distinct on and off time periods, resulting in heterogeneous mRNA levels among single cells in a population (Golding, et al. 2005, Muramoto, et al. 2012, Raj, et al. 2006, Taniguchi, et al. 2010). Such work has led to a hypothesis that gene expression heterogeneity is a ‘bet-hedging’ mechanism that allows bacterial populations to survive a wide range of environments (Ackermann 2015). In Escherichia coli, the transcriptional noise of each promoter has been characterized (Silander, et al. 2012). It is shown that different promoters exhibit various levels of heterogeneity, and interestingly, stress response promoters appear to be the most heterogeneous. This provides further evidence that heterogeneity of gene expression is important for bacterial adaptation to various stress conditions.
Transcription is the first step in gene expression, but is not fully responsible for the ultimate levels of proteins in the cell. As such, heterogeneity of transcription may not be fully representative of the phenotypic heterogeneity observed in bacterial populations. Changes in mRNA stability, translation, and protein stability within individual cells are also expected to play important roles in defining gene expression noise and consequently the phenotypic heterogeneity. However, it has been challenging to experimentally measure these sources of gene expression noise due to underlying fluctuations in transcription. Additionally, a variety of more-subtle mechanisms can affect transcript levels, including sub-cellular localization of mRNA and ribonucleases (Redder 2016), further complicate investigations into the heterogeneity of transcription.
As the last step in gene expression, changes in protein synthesis can have a large impact on cell physiology. For instance in Salmonella, decrease in protein synthesis via treatment with ribosomal inhibitors results in drastic changes in gene expression and bacterial behavior (Brunelle, et al. 2014). At the single cell level, altering protein synthesis rates has been shown to generate physiologically distinct subpopulations, e.g., bacterial persisters due to increased HipA toxin activity that decreases translation (Germain, et al. 2015). Changes in ribosomal stalling and rescue affect both translation and mRNA stability (Hayes and Sauer 2003, Jin, et al. 2016), however whether this affects single cell heterogeneity in a population is currently unknown.
In addition to changes in protein synthesis rate, changes in translational fidelity also affect cell behavior. Incorporation of noncognate amino acids into proteins leads to alteration of protein activity or activation of stress responses, which can positively or negatively impact cell physiology (Fan, et al. 2017b, Pan 2013, Pouplana, et al. 2014, Reynolds, et al. 2010). In the past decade, there has been increasing evidence that translational fidelity is regulated by environmental stresses, such as nutrient starvation (Ballesteros, et al. 2001, Zaborske, et al. 2014), oxidative stress (Bullwinkle, et al. 2014, Ling and Söll 2010, Netzer, et al. 2009), and temperature shift (Meyerovich, et al. 2010, Schwartz and Pan 2016), and that increased translational errors facilitate bacterial survival under severe oxidative and antibiotic stresses (Fan, et al. 2015, Javid, et al. 2014, Su, et al. 2016). To understand the heterogeneity of translational fidelity and its role in environmental adaptation, sensitive reporters to quantitate translational errors in individual cells are needed, which have been recently developed by several groups for stop codon readthrough (Fan, et al. 2017a), frameshifting (Fan, et al. 2017a, Rakauskaite, et al. 2011), and missense errors (Gomes, et al. 2016, Su, et al. 2016).
To separate transcriptional noise from translational noise, we have developed a dual-fluorescent reporter fusion system to measure levels of stop codon readthrough and frameshifting errors in single bacterial cells (Fan, et al. 2017a). Like transcription rates, ribosomal accuracy is also shown to be heterogeneous in bacterial populations. Interestingly, this reporter system has also revealed that increased errors during protein synthesis may positively affect a subpopulation of cells, as cells with higher UGA stop codon readthrough recover from stationary phase faster than cells with low read-through.
The molecular mechanisms that affect the heterogeneity of stop codon readthrough are still not fully understood. When a ribosome encounters a stop codon in E. coli, the codon is recognized by release factor 1 (RF1) or 2 (RF2), then release factor 3 catalyzes the release of the ribosome and RF1 or RF2 complex from the mRNA (Youngman, et al. 2008). Meanwhile, charged tRNAs can compete with the release factors and allow further elongation along the mRNA. Population-based experiments suggest that increased levels of tRNA enhance stop codon readthrough (Fan, et al. 2017a). It is reasonable to hypothesize that variation in the levels of tRNAs and release factors among single cells accounts for the heterogeneity of stop codon readthrough in a population, which needs to be directly tested in future studies.
The current reporter systems for translational fidelity contain several technical limitations. For example, translational errors that have very low rates, such as most missense errors and readthrough of more stringent stop codons like UAA, cannot be quantified in individual cells at present. Also, quantitation of multiple types of errors within a single cell remains challenging, due to the limited number of orthogonal fluorescence reporters currently available. The development of more sensitive and versatile fluorescence reporters is therefore warranted.
Increasing evidence suggests that phenotypic heterogeneity within communities is critical for bacterial adaption to environmental changes. The recently developed tools to quantitate translational errors in single cells can be applied to address many key questions in future studies. For instance, what causes noisy translational errors in single cells? How do environmental conditions affect the level and heterogeneity of translational errors? What is the role of translational fidelity during bacteria-host interaction? We are therefore entering an exciting new era of understanding the physiological impact of translational fidelity.
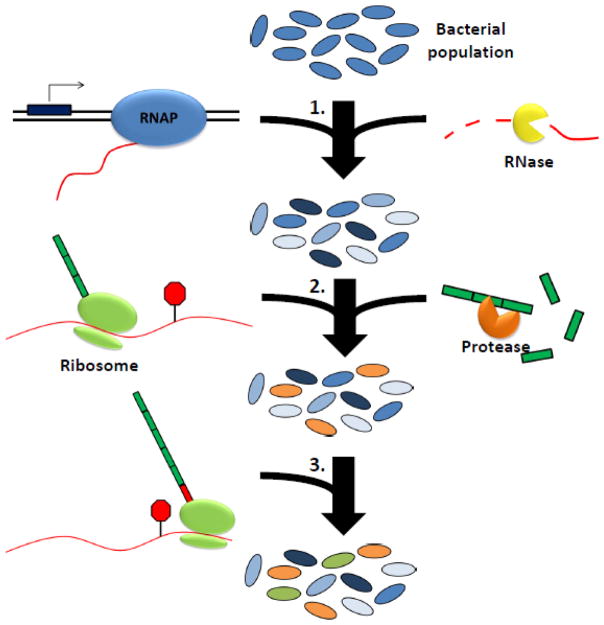
Figure 1. Noise in gene expression impacts phenotypic heterogeneity.
Fluctuation of the protein levels among individual cells of the genetically-identical population is a cumulative result from gene expression noise at various steps, including transcription by the RNA polymerase (RNAP), mRNA degradation, translation, and protein degradation. Gene expression noise contributes to the phenotypic heterogeneity that diversifies individual cells and provides a bet-hedging mechanism for the population to quickly adapt to changing environments.
Acknowledgments
This work was funded by NIGMS R01GM115431 (J.L.).
References
- Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- Ballesteros M, Fredriksson A, Henriksson J, Nystrom T. Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J. 2001;20:5280–5289. doi: 10.1093/emboj/20.18.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle BW, Bearson BL, Bearson SM. Chloramphenicol and tetracycline decrease motility and increase invasion and attachment gene expression in specific isolates of multidrug-resistant Salmonella enterica serovar Typhimurium. Front Microbiol. 2014;5:801. doi: 10.3389/fmicb.2014.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullwinkle T, Reynolds NM, Raina M, Moghal AB, Matsa E, Rajkovic A, Kayadibi H, Fazlollahi F, Ryan C, Howitz N, Faull KF, Lazazzera B, Ibba M. Oxidation of cellular amino acid pools leads to cytotoxic mistranslation of the genetic code. eLife. 2014:e02501. doi: 10.7554/eLife.02501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Fan Y, Evans CR, Barber KW, Banerjee K, Weiss KJ, Margolin W, Igoshin OA, Rinehart J, Ling J. Heterogeneity of stop codon readthrough in single bacterial cells and implications for population fitness. Mol Cell. 2017a;67:826–836. e825. doi: 10.1016/j.molcel.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Evans CR, Ling J. Rewiring protein synthesis: From natural to synthetic amino acids. Biochim Biophys Acta. 2017b doi: 10.1016/j.bbagen.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Wu J, Ung MH, De Lay N, Cheng C, Ling J. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res. 2015;43:1740–1748. doi: 10.1093/nar/gku1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain E, Roghanian M, Gerdes K, Maisonneuve E. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci USA. 2015;112:5171–5176. doi: 10.1073/pnas.1423536112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Gomes AC, Kordala AJ, Strack R, Wang X, Geslain R, Delaney K, Clark WC, Keenan R, Pan T. A dual fluorescent reporter for the investigation of methionine mistranslation in live cells. RNA. 2016;22:467–476. doi: 10.1261/rna.054163.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CS, Sauer RT. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol Cell. 2003;12:903–911. doi: 10.1016/s1097-2765(03)00385-x. [DOI] [PubMed] [Google Scholar]
- Javid B, Sorrentino F, Toosky M, Zheng W, Pinkham JT, Jain N, Pan M, Deighan P, Rubin EJ. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc Natl Acad Sci USA. 2014;111:1132–1137. doi: 10.1073/pnas.1317580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Jin S, Wu W. Regulation of bacterial gene expression by ribosome stalling and rescuing. Curr Genet. 2016;62:309–312. doi: 10.1007/s00294-015-0545-3. [DOI] [PubMed] [Google Scholar]
- Ling J, Söll D. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc Natl Acad Sci USA. 2010;107:4028–4033. doi: 10.1073/pnas.1000315107. 1000315107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovich M, Mamou G, Ben-Yehuda S. Visualizing high error levels during gene expression in living bacterial cells. Proc Natl Acad Sci USA. 2010;107:11543–11548. doi: 10.1073/pnas.0912989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Cannon D, Gierlinski M, Corrigan A, Barton GJ, Chubb JR. Live imaging of nascent RNA dynamics reveals distinct types of transcriptional pulse regulation. Proc Natl Acad Sci USA. 2012;109:7350–7355. doi: 10.1073/pnas.1117603109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer N, Goodenbour JM, David A, Dittmar KA, Jones RB, Schneider JR, Boone D, Eves EM, Rosner MR, Gibbs JS, Embry A, Dolan B, Das S, Hickman HD, Berglund P, Bennink JR, Yewdell JW, Pan T. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature. 2009;462:522–526. doi: 10.1038/nature08576. nature08576 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T. Adaptive translation as a mechanism of stress response and adaptation. Annu Rev Genet. 2013;47:121–137. doi: 10.1146/annurev-genet-111212-133522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouplana LR, Santos MA, Zhu JH, Farabaugh PJ, Javid B. Protein mistranslation: friend or foe? Trends Biochem Sci. 2014;39:355–362. doi: 10.1016/j.tibs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakauskaite R, Liao PY, Rhodin MH, Lee K, Dinman JD. A rapid, inexpensive yeast-based dual-fluorescence assay of programmed--1 ribosomal frameshifting for high-throughput screening. Nucleic Acids Res. 2011;39:e97. doi: 10.1093/nar/gkr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redder P. How does sub-cellular localization affect the fate of bacterial mRNA? Curr Genet. 2016;62:687–690. doi: 10.1007/s00294-016-0587-1. [DOI] [PubMed] [Google Scholar]
- Reynolds NM, Lazazzera BA, Ibba M. Cellular mechanisms that control mistranslation. Nat Rev Microbiol. 2010;8:849–856. doi: 10.1038/nrmicro2472. nrmicro2472 [pii] [DOI] [PubMed] [Google Scholar]
- Schwartz MH, Pan T. Temperature dependent mistranslation in a hyperthermophile adapts proteins to lower temperatures. Nucleic Acids Res. 2016;44:294–303. doi: 10.1093/nar/gkv1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silander OK, Nikolic N, Zaslaver A, Bren A, Kikoin I, Alon U, Ackermann M. A genome-wide analysis of promoter-mediated phenotypic noise in Escherichia coli. PLoS Genet. 2012;8:e1002443. doi: 10.1371/journal.pgen.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HW, Zhu JH, Li H, Cai RJ, Ealand C, Wang X, Chen YX, Kayani MU, Zhu TF, Moradigaravand D, Huang H, Kana BD, Javid B. The essential mycobacterial amidotransferase GatCAB is a modulator of specific translational fidelity. Nat Microbiol. 2016;1:16147. doi: 10.1038/nmicrobiol.2016.147. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman EM, McDonald ME, Green R. Peptide release on the ribosome: mechanism and implications for translational control. Annu Rev Microbiol. 2008;62:353–373. doi: 10.1146/annurev.micro.61.080706.093323. [DOI] [PubMed] [Google Scholar]
- Zaborske JM, DuMont VL, Wallace EW, Pan T, Aquadro CF, Drummond DA. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biol. 2014;12:e1002015. doi: 10.1371/journal.pbio.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]