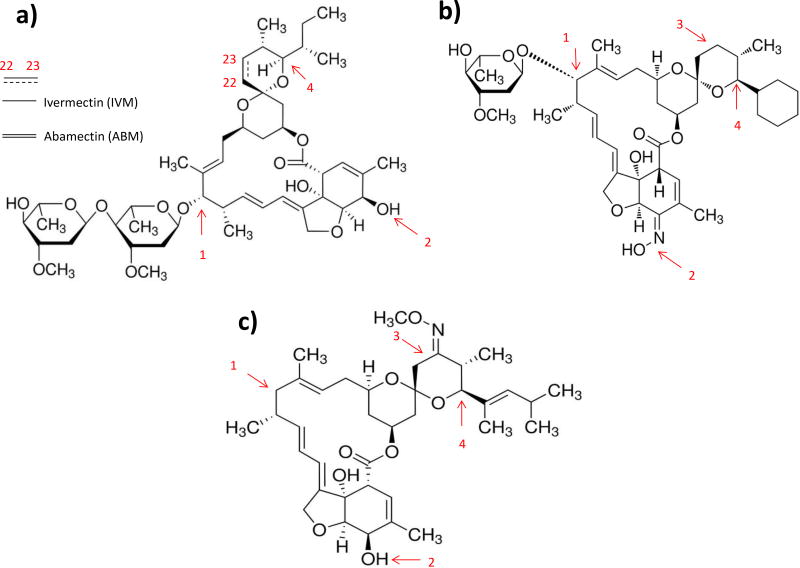
Figure 1.
Structural differences between IVM or ABM (a), SEL (b) and MOX (c) may play an important role in determining their pharmacological effects and pharmacokinetic properties. ABM possesses a double bond at C22–C23 position of spiroketal unit and IVM is an outcome of chemical hydrogenation of ABM (a). 1) IVM possesses two saccharide moieties; SEL contains one saccharide moiety and MOX does not have any saccharide moiety. 2) SEL has an oxime group instead of the hydroxyl group of the tetrahydro-benzofuran unit in IVM, ABM and MOX. 3) MOX contains a methoxime group at C23 position and this position is hydrogenated in remaining avermectins. Lastly, 4) IVM and ABM has isopropyl/isobutyl group, SEL has a cyclohexyl ring and MOX contains a dimethyl-butyl group at C25 position.