Abstract
Although cerebrovascular factors are the second leading cause of cognitive impairment and dementia in elderly, the precise spatial and temporal trajectories of vascular decline in aging have not been fully characterized. With an advanced cerebrovascular reactivity (CVR) MRI technique that specifically informs vascular stiffness and dilatory ability of cerebral vessels, we present four-year longitudinal CVR data measured in 116 healthy individuals (20–88 years of age). Our data revealed a spatial heterogeneity in vascular decline in aging (p=0.003), in that temporal lobe showed the fastest rate of longitudinal CVR decline, followed by parietal and frontal lobes. The rate of CVR decline was also age-dependent. Middle age, not older age, manifested the fastest rate of longitudinal CVR decline (p<0.05). Longitudinal changes in CVR were associated with changes in processing speed (p=0.031) and episodic memory (p=0.022), but not with working memory or reasoning. The rate of longitudinal CVR change was not different between hypertensive and normotensive participants. However, cross-sectionally, individuals with hypertension revealed in a lower CVR compared to normotensive participants (p=0.016). These findings help elucidate age-related decline in brain hemodynamics and support CVR as a non-invasive biomarker in evaluating cerebrovascular conditions in elderly individuals.
Keywords: age, BOLD, cerebrovascular reactivity, cognition, hypertension, hypercapnia
Introduction
Although vascular factors are the second most common cause of dementia in older individuals (Kivipelto et al., 2001), characteristics of brain vascular changes that occur with aging are still underspecified and poorly understood. Most studies of vascular insults that occur with advanced age have investigated white matter hyperintensities on T2 weighted MRI (Spilt et al., 2005). These studies have found WMH’s increase dramatically at advanced age (Valdes Hernandez Mdel et al., 2013). However, vascular changes in the gray matter, where most neurocomputation takes place, have not been characterized to date due to the fact that few WMH occur in gray matter. Furthermore, structural lesions such as WMH are late events and are generally only observed at an age of 50 years and above (Debette and Markus, 2010), and thus are likely insensitive to any vascular changes that occur in the middle age.
One of the most important functions of cerebral vessels is to dilate and constrict dynamically in response to changes in resource demands on the vascular system. Thus, a vasodilatory index, referred to as cerebrovascular reactivity (CVR), can provide a direct assessment of efficient cerebrovascular function (Lu et al., 2011; Tancredi et al., 2012). Age-related alterations in CVR have been reported (Coverdale et al., 2016; De Vis et al., 2015; Gauthier et al., 2013; Kastrup et al., 1998; Lu et al., 2011; Schieve and Wilson, 1953). CVR was found to decrease significantly with age, at a rate that was faster than that of perfusion (Lu et al., 2011). One limitation of the prior studies is that they were cross-sectional in design and only measured age-related CVR differences across subjects and were unable to determine the rate of decline within subjects, and whether rate of decline varied by age or brain region.
Furthermore, it is important to determine if measurement of brain vascular markers such as CVR can predict cognition. If the relationship between CVR and cognitive function can be established, it would further support the potential of CVR as a biomarker in the diagnosis and treatment monitoring of conditions such as vascular cognitive impairment. Prior studies have suggested that processing speed is one of the first cognitive domains affected by cerebrovascular dysfunction (Knopman et al., 2015; Selnes et al., 2009), but, to date, there is no evidence whether change in CVR track with decline in cognition.
A central goal of this study was to elucidate the magnitude of change in CVR over a four-year interval in 116 cognitively-normal adults ranging from 20 to 88 years old who were participants in the Dallas Lifespan Brain Study. The study appears to be the first longitudinal study that characterizes both the spatial and temporal properties of CVR. We assessed whether the rate of CVR decline were similar across the brain or rather showed evidence for spatial heterogeneity, differing across major brain lobes. Temporally, We examined age-dependence of the decline rate to identify the period in adult lifespan when CVR decline was the most rapid. Longitudinal changes in CVR were compared to changes in cognitive function in the domains of processing speed, reasoning, working memory, and episodic memory. The CVR data were also analyzed in terms of their association with vascular risk of hypertension.
Material and methods
Participants
The data were collected as part of a longitudinal aging study, the Dallas Lifespan Brain Study (DLBS). The entire cohort of the DLBS contained 350 participants aged from 20 to 89 years old at Wave 1 (i.e. baseline), with 50 participants in each decade range. Of these, 205 subjects (123 female, 82 male, aged 20–88) elected to receive the CVR MRI scan at Wave 1, which requires a brief inhalation of CO2 gas mixture inside the MRI scanner. Only these subjects were invited to participate in the Wave 2 (i.e. follow-up) CVR procedures, and 116 (78 female, 38 male, aged 20–88 at Wave1) of them completed Wave 2 CVR. The participants who did not return for Wave 2 were not different from those who returned in terms of age range (p=0.94), education (p=0.83), or prevalence of hypertension (p=0.58), but were found to have a lower mini-mental state exam (MMSE) (p=0.01). There were also more male participants in the group who did not return (p=0.02). The interval between the two waves was 4.1±0.2 (range 3.5–4.7) years. The participants did not report any pulmonary, respiratory, neurologic, or psychiatric disorders according to self-completed questionnaires. None of the participants were smokers or had asthma. All participants were considered free of mild cognitive impairment or dementia at year 4 follow-up. The lowest MMSE score of the participants was 25 (N=2). The highest score of modified Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) was 12 (N=1). Demographic information of the participants is summarized in Table 1. The study was performed in accordance with the local institutional review board, and written informed consent was obtained from all participants.
Table 1.
Demographic information of the participants. p values indicate the comparisons between participants who did not return for Wave 2 and those who returned.
| Cross-sectional cohort (N=205) | Longitudinal cohort (N=116) | p value | |||||
|---|---|---|---|---|---|---|---|
| Wave 1 | Wave 1 | Wave 2 | |||||
| Female | Male | Female | Male | Female | Male | ||
| Number of subject recruited | 123 | 82 | 78 | 38 | 78 | 38 | |
| Age (y/o, mean±SD) | 50.2±18.9 | 51.5±20.6 | 51.1±19 | 49.6±17.1 | 55.2±18.9 | 53.7±17.1 | 0.94 |
| Education (years, mean±SD) | 16.3±2.4 | 16.6±2.4 | 16.4±2.3 | 16.4±2.3 | 16.4±2.3 | 16.4±2.3 | 0.83 |
| Mini-Mental State Exam (mean±SD) | 28.6±1.2 | 28±1.4 | 28.6±1.2 | 28.4±1.2 | 29.4±1.1 | 29.3±1.1 | 0.01 |
| Number of subjects with hypertension history (percentage) | 18 (15%) | 17 (21%) | 10 (13%) | 8 (21%) | 10 (13%) | 8 (21%) | 0.58 |
Hypertension status was obtained by a self-reporting questionnaire. The questionnaire asks: 1) “Do you have a history of hypertension (high blood pressure)?” and 2) “Do you have hypertension (high blood pressure) now?”. The participants who answered Yes to both questions were considered a sub-group, HTNcurrent, who are currently hypertensive. Those who answered Yes to the first question but No to the second question were considered another sub-group, HTNpast, who has a past history of hypertension but is not currently hypertensive, presumably through effective control by medication. CVR were compared across the sub-groups of participants.
CVR MRI
All experiments were performed on a 3T MR system (Philips Healthcare, Best, The Netherlands). Wave 1 and Wave 2 studies used identical protocols. The volunteers were all fitted with foam pads to reduce head motion. CVR was measured using a hypercapnia challenge established in previous studies (Liu et al., 2014; Lu et al., 2011; Marshall et al., 2014; Yezhuvath et al., 2009). Briefly, each subject was fitted with a nose clip and a mouthpiece to ensure mouth breathing. Hypercapnia (5% CO2 mixed with 21% O2 and 74% N2) was administered through a plastic bag with a valve to switch between room air and CO2 mixture. A research assistant was inside the magnet room throughout the experiment to switch the valve and monitor the subject. The subject was instructed to establish a breathing rhythm and try to maintain it throughout the scan. The subject inspired room air and the hypercapnic gas in an interleaved manner similar to a block design fMRI experiment, which was 1-min room air inhalation followed by 1-min CO2 inhalation, repeated three times with additional 1-min room air inhalation at the end. The total duration was 7 minutes. This paradigm was previously shown to provide comparable results to paradigms that used longer duration (e.g. 4 mins) of CO2 breathing (Yezhuvath et al., 2009), with a better tolerability. Blood oxygenation level-dependent (BOLD) MR images were acquired during this period. The BOLD imaging parameters were field of view (FOV) = 220×220 mm2, matrix size = 64×64, 43 axial slices, thickness = 3.5 mm, no gap, TR/TE/flip angle=200ms/25ms/80 °, and single-shot EPI. A high-resolution T1-weighted scan was acquired with the following parameters: magnetization-prepared rapid acquisition of gradient echo sequence, TR/TE/TI=8.1 ms/3.7 ms/1100 ms, shot interval 2100 ms, flip angle=18°, voxel size=1×1×1 mm3, number of slices 160, sagittal slice orientation, and duration 3 min and 57s. Physiologic parameters, including end-tidal (Et) CO2, breathing rate, heart rate and arterial oxygenation (Ya) were recorded during the experiment (MEDRAD; Novametrix Medical Systems).
CVR Data Processing
CVR data were processed with a general linear model (SPM, University College London, UK), in which the dependent variable was BOLD signal and the regressor was the EtCO2 time course. CVR in units of %BOLD signal change per mmHg of EtCO2 change (%BOLD/mmHg CO2) was obtained.
The CVR maps were co-registered to the individual high-resolution T1-weighted image, followed by normalization to the Montreal Neurological Institute (MNI) template space. CVR values for major brain lobes, including frontal, temporal, parietal, and occipital, as well as whole-brain, were obtained with a parcellated brain template (Tzourio-Mazoyer et al., 2002).
Cognitive Assessment
Four domains of cognitive function were assessed, including processing speed, working memory, reasoning, and episodic memory. Processing speed was measured using the Digit Comparison Task, adapted from Letter Comparison Task of Salthouse & Babcock (Salthouse, 1991), and WAIS-III Digit Symbol (Wechsler, 1997). Working memory was evaluated from the Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Working Memory (Robbins et al., 1994) and WAIS-III Letter Number Sequencing (Wechsler, 1997). Reasoning was estimated using the Raven’s progressive matrices (Raven, 1998), ETS letter sets (Ekstrom, 1976) and CANTAB Stockings of Cambridge (Robbins et al., 1994). Episodic memory was assessed using the modified Hopkins Verbal Learning Task (HVLT) (Brandt, 1991) and the CANTAB (Robbins et al., 1994) Verbal Recognition Memory Task. The details of the procedures for the cognition tests can be found in the Supplementary Material.
Standardized z-scores were calculated for each test (an individual’s test score minus mean test score divided by standard deviation). Composite measures for each of the four cognitive domains were created by adding the z-scores for all tests assigned to each domains and dividing by the number of tests contributing to that cognitive domain. To evaluate longitudinal changes in cognition, we standardized the scores for each cognitive test by pooling the two scores (Wave 1 and Wave 2) of all participants and applying a z-transformation, and then for each cognitive domain, computed the composite measures at Wave 1 and Wave 2 separately. The difference from Wave 1 to Wave 2 was the longitudinal change score in that cognitive domain.
Statistical analysis
For the first analysis, we investigated the effects of age and test wave on whole-brain CVR in a combined general linear model. Age was treated as a continuous variable and Wave (1 or 2) was treated as a repeated measure with whole-brain CVR as the dependent variable.
The second analyses focused on the rate of CVR change, calculated as (Wave 2 CVR-Wave 1 CVR)/time gap between waves, for individual brain lobes (frontal, parietal, temporal, and occipital). Mixed-design analysis of variance (ANOVA) was performed, treating lobe as a within-subject variable, and age and age2 as between-subject variables. The inclusion of both the linear and quadratic terms allows for the assessment of any age-related non-linearity in CVR decline in any lobe. If a significant lobe effect was observed, we further compared pairs of brain lobes using paired Student t-test. If a significant age-related interaction was observed, we tested the age relationship for each lobe separately in the post hoc analyses.
Then, to understand the relationship between CVR and cognition, we used a general linear model that included, as variables of interest, both linear and quadratic components for age, change in CVR, and their interactions. Additional control variables included baseline CVR and baseline cognitive performance.
Finally, to examine whether baseline CVR and CVR change may be predictive of hypertension, two regression analyses were performed, using baseline CVR or CVR change as the variable of interest to predict hypertension groups, while controlling for age. In all tests, a p value of 0.05 or less was considered significant.
Results
Dependence of whole-brain CVR on age and wave index
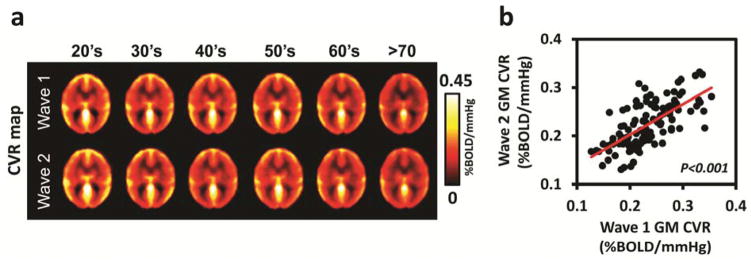
The first analysis examined the evidence for longitudinal change in CVR across the adult lifespan. A multivariable linear model treated age as a continuous variable and wave index (i.e. 1 for baseline and 2 for follow-up) as a repeated measure. The age effect was modeled for both linear and quadratic effects. Results indicated that, for whole-brain CVR, the Age, but not Age2, was significant and occurred because whole-brain CVR decreased significantly with increased age (p<0.001). The Wave effect was also significant because CVR decreased from Wave 1 to Wave 2 (p=0.002). The Age x Wave interaction did not approach significance for either the linear or quadratic effect. Figure 1a depicts the systematic age-related decrease in CVR from the 20’s through the 70’s for both Wave 1 and 2. Annual change rate for the whole-brain CVR across the four years was −0.0024±0.0009 %BOLD/mmHg/year (mean±SE), which is equivalent to −0.99±0.39%/year in relative units. Figure 1b displays scatterplot of whole-brain gray matter (GM) CVR between Wave 1 and Wave 2. Cross correlation coefficient between CVR values of the two Waves was 0.67 (p<0.001).
Figure 1.
CVR at Wave 1 and Wave 2. (a) Decade-by-decade averaged maps of CVR for Wave 1 and Wave 2. (b) Scatter plot between whole brain gray matter (GM) Wave 1 and Wave 2 CVR values. Each dot represents data from one subject (N=116). Pearson’s correlation coefficient = 0.67 (p<0.001).
Longitudinal changes in CVR across brain lobes
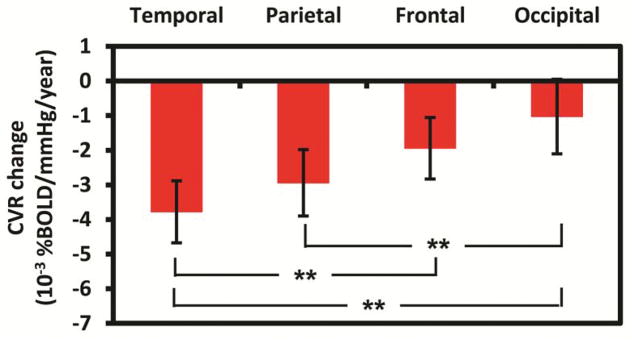
For the next analysis, we assessed whether the CVR change differed across brain lobes and whether there were age-related interactions. The analysis yielded a significant main effect of lobe (p=0.003) on the rate of CVR declines. Figure 2 shows that CVR decline rate varied across the major brain lobes. Comparisons indicated that the temporal lobe showed a faster decline rate than the frontal (p=0.001) and occipital lobes (p<0.001), with a trend-level effect towards faster decline compared to the parietal lobe (p=0.087). Additional comparisons indicated that parietal lobe had a faster decline rate than occipital lobe (p<0.001), with a trend difference when compared to the frontal lobe (p=.098). The occipital lobe was found to have the slowest rate of CVR decline among the major brain lobes investigated, and the decline rate was not significantly different from zero (p=0.344, Figure 2).
Figure 2.
The rate of CVR change in major brain lobes. The error bars indicate standard errors. **: p<0.001.
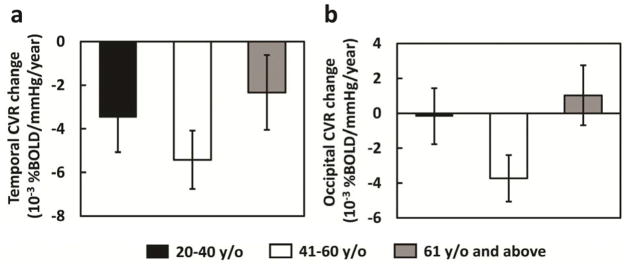
The linear component of the Age x Lobe interaction was not significant (p=0.304), but the quadratic component was significant (Age2 x Lobe interaction, p=0.011). The quadratic effect applied only to the temporal (p=0.027, Figure 3 left panel) and occipital lobes (p=0.015, Figure 3 right panel), whereas the rate of decline in CVR was unaffected by age for the frontal (p=.406) and parietal lobes (p=.162). In sum, the analysis showed the temporal lobe was most sensitive to decline in CVR and that the greatest decline occurred during middle age.
Figure 3.
Bar plots of longitudinal CVR decline rate stratified by age groups. The total of 116 participants were divided into three age ranges, 20–40 years old (N=36), 41–60 years old (N=41), and 61 and above (N=39). The CVR decline rate showed a quadratic dependence on age in temporal lobe (left panel, p=0.027) and (b) occipital lobe (right panel, p=0.015). The error bars indicate standard errors.
Longitudinal CVR change and cognitive change
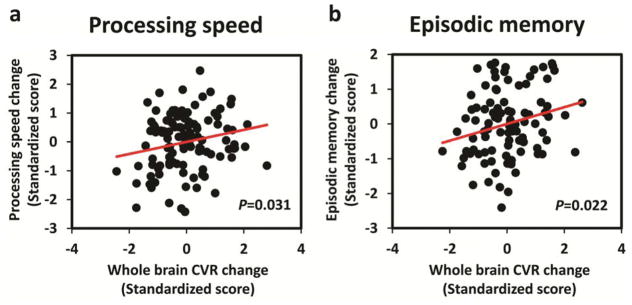
For the next set of analyses, we used the general linear model described earlier to examine whether changes in CVR across the four-year interval were predictive of cognitive decline during the same period. First, we examined whether whole brain CVR decline predicted changes in four domains of cognition. Results indicated that whole brain CVR decline significantly predicted declines in processing speed (p=0.031, Figure 4a) and episodic memory (p=0.022, Figure 4b). Based on these findings, we looked at whether the effects for processing speed and episodic memory were qualified by brain lobe. Results indicated that change in CVR in the temporal lobe (p=0.033) and the frontal lobe (p=0.039) predicted change in processing speed. Moreover, both the parietal (p=0.080) and occipital lobes (p=0.055) showed marginal significance. This widespread CVR change effect on processing speed was consistent across the lifespan since no age interaction was found in any lobes (p>0.1).
Figure 4.
Association between longitudinal changes (over a four-year period) in cognition and whole brain GM CVR. (a): processing speed. (b): episodic memory.
Similarly, the relationship between CVR change and episodic memory change was also widespread across lobes (temporal: p=0.019, occipital: p=0.020, parietal: p=0.053). There was a significant Age x CVR change interaction on memory change in the occipital lobe (p=0.029) such that the CVR declines were more predictive of memory declines relationship in younger adults.
The relationship between CVR and hypertension
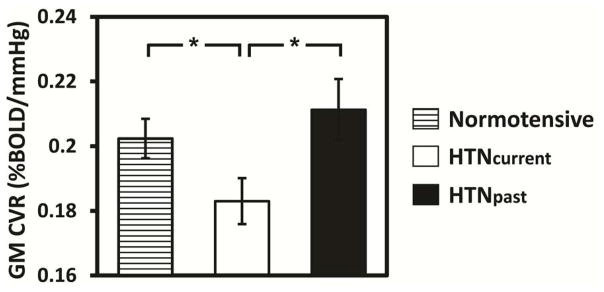
We assessed both the baseline relationship, as well as the longitudinal change in CVR, to hypertension. A total of 21 participants (mean age 68.1±13.5) reported themselves to be currently hypertensive (HTNcurrent), 20 of whom were taking anti-hyptertensive medications. An additional 14 participants (age 62.2±19.1) reported that they had a past history of hypertension but were not currently hypertensive (HTNpast), none of whom were taking anti-hypertensive medications. These sub-groups were compared to an age-matched (55 years and above) normotensive group (N=58, age 69.3±8.9). As expected, the lowest baseline CVR occurred in the current hypertensives. Both the past hypertensives and normotensives had significantly higher CVR than the current hypertensives (p=0.016 and 0.025 respectively), and did not differ significantly from each other (Figure 5). Longitudinal change in CVR was not associated with hypertensive status.
Figure 5.
Comparison of whole brain GM CVR among normotensive subjects (N=58), subjects who are currently hypertensive (HTNcurrent) (N=21), and subjects who have a past history of hypertension (HTNpast) (N=14). The error bars indicate standard errors. *: p<0.05.
Discussion
This study presented longitudinal changes in cerebrovascular reactivity over four years in an across adult lifespan sample, and examined its relationship to cognition. Previous studies have reported that WMH volume increases with age (Spilt et al., 2005) and that CBF decreases with age (Chen et al., 2011; Lu et al., 2011). However, less has been known about longitudinal change in cerebrovascular reactivity. The design of the present study provides longitudinal data about cerebrovascular change in young, middle age, and older adults. Since CVR measures vasodilatory capacity of blood vessels, the observed changes in the present study likely reflect a thickening and hardening of the walls, as well as narrowing of the vessels.
We report the following major findings. 1) There is detectable decline in CVR over four years across the lifespan. 2) The temporal lobe revealed the fastest decline in cerebrovascular reactivity, while the occipital lobe was the most preserved. 3) The greatest rate of CVR decline occurred in the temporal lobe during middle age. 4) Decline in cerebrovascular reactivity is related to declines in cognition, particularly processing speed and episodic memory.
In support of our first finding, we initially used the whole brain to calculate longitudinal change in global CVR and found evidence for significant decline in CVR between wave 1 and wave 2, as well as age-differences previously reported (Lu et al., 2011). However, cerebrovascular changes are known to be heterogeneous across brain regions. For example, occipital lobe has been shown to have both the least amount of longitudinal WMH and volumetric shrinkage (Raz et al., 2005; Raz et al., 2012), as well as relatively preserved hemodynamic function (D’Esposito et al., 1999). Additionally, previous studies on CBF have shown that resting brain perfusion shows the most pronounced age effect in the frontal lobe (Beason-Held et al., 2009; Chen et al., 2011; Lu et al., 2011), particularly in the prefrontal cortex.
Hence, we examined change in CVR as a function of brain lobe. The analysis provided the evidence that occipital lobe had the least change in cerebrovascular function, whereas the fastest CVR decline was observed in the temporal lobe (followed by parietal lobe). Importantly, the analysis by lobe revealed that there were age differences in rate of change. Surprisingly, the greatest decline in CVR did not occur in old age, but rather in middle age, a finding that could not be detected without a lifespan longitudinal design. These findings are similar to those reported by Raz et al. (Raz et al., 2012) who found changes in WMH volume over a period of 30 months, and showed that the rate of WMH volume expansion in middle-aged adults was greater than that at older ages.
Examination of the effect of hypertension on CVR revealed that hypertension is associated with a lower CVR, suggest that CVR may be a useful biomarker in indexing the impact of systemic vascular risks on the brain. More interestingly, we observed that individuals with a history of hypertension, but whose BP is well controlled by medication, had a significant higher CVR than those whose BP is poorly controlled. This finding is in good agreement with reports of Muller et al. (Muller et al., 2012) that global blood flow declines faster in untreated or poorly controlled hypertensive individuals than those with controlled hypertension. A possible mechanism is that vascular smooth muscle tone is mediated by nitric oxide (NO) derived from endothelial cells and neuron (Faraci and Brian, 1994) and patients with hypertension are characterized by reduced bioavailability of NO, leading to reduced CVR (Bomboi et al., 2010). With antihypertensive medication, improved endothelial function and NO formation have been shown in animal studies (Oyama et al., 2010). The observation in the present study provides evidence that CVR is a modifiable imaging biomarker, as compared to WMH which is thought to be irreversible (Debette and Markus, 2010). These features afford CVR a potential endpoint in clinical trials of cerebrovascular conditions.
Although vascular dementia is the second leading cause of cognitive disability in older adults (after Alzheimer’s type), associations between cerebrovascular biomarkers and cognition have not been well studied. In a cross-sectional study with a large sample of 1906 older participants (age 76±5 years), Knopman and colleagues (Knopman et al., 2015) reported that higher WMH volume was most significantly associated with deficits in processing speed, but not with memory. Our longitudinal CVR data revealed a similar association between changes in CVR and processing speed, the most sensitive cognitive marker of aging (Park et al., 2002; Salthouse, 1996), using a sample size that was substantially smaller. Not only was there a global relationship in whole brain CVR to processing speed, significant associations were found in both temporal and frontal lobes, with marginal significance in the remaining two. Of equal importance was the finding that declines in CVR also track significantly with decline in episodic memory in temporal, parietal and occipital lobes, a finding not reported by Knopman (2015) for WMH. Therefore, it is plausible that CVR may be a more sensitive vascular biomarker than WMH in predicting cognitive changes. These findings also suggest that processing speed and episodic memory are among the most vulnerable cognitive domains to cerebrovascular dysfunction.
Finally, the findings from the present study highlight the importance of studying the entire adult lifespan to understand the effects of aging on the brain. Because middle aged adults have rarely been subjects of most brain imaging studies, particularly longitudinal studies, we know little about when exactly in adulthood brain changes occur. The Dallas Lifespan Brain Study is providing increasing evidence that there are important differences during middle age in brain function (Chan et al., 2014; Park et al., 2013).
Strengths of our study include a large sample size (116 participants), that it has four-year longitudinal follow-up data, and that data of all participants and time points were collected at a single-site on one MRI system, which ensures data harmonization and compatibility. Our cognitive tests were comprehensive and included multiple cognitive domains with each domain consisting of multiple tasks.
Several limitation of current study should be acknowledged. First, the present study only examined CVR as an index of cerebrovascular function, but did not investigate corresponding changes in other vascular imaging biomarkers such as WMH volume or CBF. Future studies should compare the temporal trajectory of these different vascular parameters. A second limitation is that the follow-up period, four years, was not as long as some of the earlier studies (Gottesman et al., 2014; Nyberg et al., 2010). However, we note that the present study is the first longitudinal investigation of CVR imaging index to date, and we are currently in the process of planning for a Wave 3 study, which should manifest more longitudinal changes in both CVR and cognition. Nevertheless, we still observed longitudinal decline in CVR over this relatively short period, and its association with cognitive decline widespread across the brain, which may suggest CVR being a more specific and sensitive vascular marker than WMH and CBF. Finally, we point out that, during our CVR experiment, we did not control for other vasoactive factors such as consumption of caffeine. This could add noise or bias to our data, although some previous studies have suggested a minimal effect of caffeine consumption on CVR measurement (Chen and Parrish, 2009).
Conclusions
The present work showed that CVR, a biomarker of cerebral vasodilatory function, decreases with age. Longitudinal decline in vascular function was most pronounced in the middle age and spatially it was most prominent in the temporal lobe. Longitudinal change in CVR is strongly associated with changes in processing speed and episodic memory, suggesting the potential of CVR imaging measure as a useful biomarker indicating cerebrovascular conditions related to cognitive changes.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers R37 AG006265, R01 AG042753, R01 MH084021, R01 NS067015, P41 EB015909, and R00 AG036848), and China Medical University (CMU104-N-11).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beason-Held LL, Kraut MA, Resnick SM. Stability Of Default-Mode Network Activity In The Aging Brain. Brain Imaging Behav. 2009;3:123–131. doi: 10.1007/s11682-008-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomboi G, Castello L, Cosentino F, Giubilei F, Orzi F, Volpe M. Alzheimer’s disease and endothelial dysfunction. Neurol Sci. 2010;31:1–8. doi: 10.1007/s10072-009-0151-6. [DOI] [PubMed] [Google Scholar]
- Brandt J. The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111:E4997–5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Parrish TB. Caffeine’s effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage. 2009;44:647–652. doi: 10.1016/j.neuroimage.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale NS, Badrov MB, Kevin Shoemaker J. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X15626156. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- De Vis JB, Hendrikse J, Bhogal A, Adams A, Kappelle LJ, Petersen ET. Age-related changes in brain hemodynamics; A calibrated MRI study. Hum Brain Mapp. 2015;36:3973–3987. doi: 10.1002/hbm.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Faraci FM, Brian JE., Jr Nitric oxide and the cerebral circulation. Stroke. 1994;25:692–703. doi: 10.1161/01.str.25.3.692. [DOI] [PubMed] [Google Scholar]
- Gauthier CJ, Madjar C, Desjardins-Crepeau L, Bellec P, Bherer L, Hoge RD. Age dependence of hemodynamic response characteristics in human functional magnetic resonance imaging. Neurobiol Aging. 2013;34:1469–1485. doi: 10.1016/j.neurobiolaging.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998;29:1311–1314. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR, Jr, Graff-Radford J, Schneider AL, Windham BG, Coker LH, Albert MS, Mosley TH, Jr Investigators AN. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lu H, Filbey FM, Tamminga CA, Cao Y, Adinoff B. MRI assessment of cerebral oxygen metabolism in cocaine-addicted individuals: hypoactivity and dose dependence. NMR Biomed. 2014;27:726–732. doi: 10.1002/nbm.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall O, Lu H, Brisset JC, Xu F, Liu P, Herbert J, Grossman RI, Ge Y. Impaired cerebrovascular reactivity in multiple sclerosis. JAMA Neurol. 2014;71:1275–1281. doi: 10.1001/jamaneurol.2014.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Sasaki T, Omura-Matsuoka E, Terasaki Y, Sugiyama Y, Sakoda S, Kitagawa K. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J Neurosci Res. 2010;88:2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank A, Park DC. An fMRI study of episodic encoding across the lifespan: changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 2013;51:448–456. doi: 10.1016/j.neuropsychologia.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JR, JC, Court JH. Manual for Raven’s Progressive Materices and Vocabulary Scales. San Antonio, TX: Harcourt Assessment; 1998. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 2012;33:429e421–425. doi: 10.1016/j.neurobiolaging.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Mcinnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (Cantab) - a Factor-Analytic Study of a Large-Sample of Normal Elderly Volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TAB, RL Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Schieve JF, Wilson WP. The influence of age, anesthesia and cerebral arteriosclerosis on cerebral vascular activity to CO2. Am J Med. 1953;15:171–174. doi: 10.1016/0002-9343(53)90067-9. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445–454. doi: 10.1016/j.athoracsur.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilt A, Geeraedts T, de Craen AJ, Westendorp RG, Blauw GJ, van Buchem MA. Age-related changes in normal-appearing brain tissue and white matter hyperintensities: more of the same or something else? AJNR Am J Neuroradiol. 2005;26:725–729. [PMC free article] [PubMed] [Google Scholar]
- Tancredi FB, Gauthier CJ, Madjar C, Bolar DS, Fisher JA, Wang DJ, Hoge RD. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2 -induced cerebrovascular reactivity. J Magn Reson Imaging. 2012;36:312–321. doi: 10.1002/jmri.23658. [DOI] [PubMed] [Google Scholar]
- del Valdes Hernandez MC, Booth T, Murray C, Gow AJ, Penke L, Morris Z, Maniega SM, Royle NA, Aribisala BS, Bastin ME, Starr JM, Deary IJ, Wardlaw JM. Brain white matter damage in aging and cognitive ability in youth and older age. Neurobiol Aging. 2013;34:2740–2747. doi: 10.1016/j.neurobiolaging.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: Thy Psychological Corporation; 1997. [Google Scholar]
- Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed. 2009;22:779–786. doi: 10.1002/nbm.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.