Abstract
Purpose
Previous studies on adrenal incidentalomas (AIs) are limited by their retrospective design, small numbers of patients, Western populations, or use of an outdated imaging technique. We investigated the characteristics of AIs in Korean patients and compared them with those reported in the largest retrospective study in Italy to discover the effects of improved imaging techniques and ethnicity differences.
Materials and Methods
This was a prospective, multicenter, cross-sectional observational study including 1005 Korean patients. Levels of plasma adrenocorticotrophic hormone, 24-h urinary free cortisol (UFC), serum cortisol after a 1 mg-dexamethasone suppression test, 24-h urinary fractionated metanephrine, and plasma aldosterone and plasma renin activity were measured. All AIs were characterized using computed tomography (CT).
Results
Compared with the results of the Italian study, AIs in Korean patients were observed more frequently in men and predominantly on the left side. Korean patients with AIs were slightly younger, and fewer patients underwent surgery. Most AIs were nonfunctional in both studies, while fewer subclinical hypercortisolism and more primary aldosteronism (PA) cases were detected in Korean patients. In our study, high UFC levels showed very low sensitivity, compared to those in the Italian study. In pheochromocytoma or PA cases, there were no hormonal differences between the studies. AIs in Korean patients were smaller, such that a lower cutoff size for detecting adrenocortical carcinoma (ACC) could be warranted.
Conclusion
Recent advances in CT technology were leveraged to provide accurate characteristics of AIs and to detect smaller ACCs.
Keywords: Adrenal incidentaloma, characteristics, Italy, Korea
INTRODUCTION
Adrenal incidentaloma (AI) is an unexpected adrenal mass discovered during a radiologic examination performed for reasons unrelated to adrenal diseases.1 Data available on the prevalence of AI are scarce and have been extrapolated from either autopsy or radiological studies. In autopsy studies, the mean AI prevalence is reported at about 2.0% (range: 1.0–8.7%).2 In radiological studies, the AI frequency is about 4% in middleaged individuals and increases to more than 10% in older adults.2 AI has become a common clinical problem and its prevalence, as determined by radiological studies, comes close to that observed upon autopsy with advances in imaging technologies, especially in the resolution of computed tomography (CT). Although the majority of AIs are nonfunctional adenomas, their differential diagnosis must include a wide range of pathologies, including those that are hyperfunctional (hormonally-active) or nonfunctional and malignant or benign.3 Moreover, recent research has focused on subclinically hyperfunctional AIs showing a mild increase in hormone levels without other signs and symptoms of relevant diseases, since these conditions can eventually influence morbidity and mortality.4,5 Accordingly, several study groups have sought to establish clinical practice guidelines for the evaluation and management of AIs.2,6,7
With the increasing significance of AIs, several studies have investigated their characteristics (e.g., number, size, site, and hormone status)8,9,10,11,12 and changes over time.13,14,15,16 However, most studies are limited by their retrospective nature, including selection bias, recall bias, and confounding factors, and small sample size. In addition, most of them were conducted in Western populations:8,9 Ethnic differences between Caucasian and East Asian populations17 may influence functional characteristics according to ethnicity-associated polymorphisms of the hormonal receptor18 and the enzyme for hormonal metabolism.19 Finally, they were conducted prior to advances in imaging, thus most AIs were screened with ultrasonography (US),8,9 which can only detect about 65% of AIs <3.0 cm.20 Therefore, to evaluate and manage AI, especially in Asians, prospective studies with larger numbers of patients using improved imaging techniques are required. To this end, we prospectively recruited patients with AIs from three Korean tertiary medical centers during a three-year period [the Co-work Of Adrenal Research (COAR) cohort]. As one of the first studies using the COAR cohort, we investigated the initial characteristics of 1005 Korean AI patients and compared them with those of the largest previous retrospective study conducted in a Study Group on Adrenal Tumors of the Italian Society of Endocrinology (SIE) cohort8 to discover the effects of improved imaging techniques and ethnicity differences.
MATERIALS AND METHODS
Study population
The study population was based on a randomized, parallelgroup, multicenter, and open-labeled trial conducted at the Asan Medical Center (AMC), Samsung Medical Center (SMC), and Konkuk University Medical Center (KUMC) in Korea (clinicaltrial. gov No. NCT01382420). From July 2011 to June 2014, we recruited 1059 consecutive patients who were newly diagnosed with AIs. Diagnosis of AI was based on the detection of adrenal masses (≥1 cm) on CT scans performed for unrelated diseases.
Patients who had previously been prescribed drugs or had diseases (i.e., thyrotoxicosis, depression, alcoholism, or rheumatologic diseases) that affect corticosteroid metabolism or cortisol secretion (n=34) were excluded. Patients who were identified to have signs or symptoms of hypercortisolism at the initial physical examination by endocrinologists (n=20) were also excluded. The remaining 1005 patients were eligible for this study.
Functional evaluation
For all patients, we measured the levels of morning plasma adrenocorticotrophic hormone (ACTH), serum cortisol, 24-h urinary free cortisol (UFC), morning serum cortisol after a 1 mgdexamethasone suppression test (1 mg-DST), serum dehydroepiandrosterone–sulfate (DHEA-S), 24-h urinary catecholamine, vanillylmandelic acid (VMA), fractionated metanephrine, plasma renin activity (PRA), and plasma aldosterone levels at baseline.
We functionally classified 1005 patients with AIs according to the following criteria, which were identical to criteria of the SIE cohort for comparison of two groups.8 Subclinical hypercortisolism (SH) was diagnosed when the patient showed at least two abnormalities among lack of suppression in the 1 mg-DST (cutoff value <5.0 µg/dL), UFC levels higher than the upper normal limit, and ACTH levels lower than 10.0 pg/mL without signs or symptoms of hypercortisolism. Pheochromocytoma was diagnosed when the patient showed elevated 24-h urinary fractionated metanephrine levels that were confirmed on the basis of histological findings. Primary aldosteronism (PA) was diagnosed when the patient showed an aldosterone-to-renin ratio above 30 that was confirmed by the lack of suppression in plasma aldosterone levels after a saline infusion test (cutoff value <5.0 ng/dL).
Imaging and histological evaluation
All AIs were characterized using CT, and an experienced radiologist at each institute reported CT findings, including number, size, attenuation, and other specific features (e.g., hemorrhage, necrosis, and calcification). Only the characteristics of the largest lesion among multiple lesions were used for analysis. Histological findings were reported by an experienced pathologist at each institute.
Adrenalectomy was randomly performed on SH patients who had at least lack of suppression in the 1 mg-DST with a cutoff value of 1.8 µg/dL, which was defined by the original trial.21 All patients biochemically diagnosed with pheochromocytoma, except for those who were lost during the follow-up period, underwent an adrenalectomy. Among PA patients, only those with an aldosterone-producing adenoma confirmed with adrenal vein sampling underwent an adrenalectomy. In addition, patients with suspected malignant adrenal masses based on CT findings (e.g., large size, heterogeneous features, or irregular shape) underwent an adrenalectomy.
Anthropometric, biochemical and, hormonal measurements
All anthropometric, biochemical, and hormonal measurements were performed at each of the three medical centers (AMC, SMC, and KUMC) in Korea.
Age, past medical history, and drug history were recorded. Height (cm) and weight (kg) were measured using a standardized protocol while subjects wore light clothing without shoes. Body mass index [BMI (kg/m2)] was calculated from their height and weight. Blood pressure (mm Hg) was recorded twice using a mercury manometer after the patient rested for more than 15 minutes, and the average value thereof was calculated. In this study, diabetes mellitus was defined as a fasting plasma glucose (FPG) level ≥126.0 mg/dL, glycated hemoglobin level (HbA1c) ≥6.5%, previous diagnosis of diabetes by a physician, or current use of anti-diabetic medications.22 Hypertension was defined as blood pressure over 140/95 mm Hg on more than two occasions, a previous diagnosis of hypertension by a physician, or current use of antihypertensive medications. We defined BMI over 25 kg/m2 as obesity.23 Lower bone mass was defined by a Z-score ≤−2.0 for premenopausal women and men aged <50 years, T-score ≤−2.5 for postmenopausal women and men aged ≥50 years,24 or patients who were taking medications for osteoporosis.
For biochemical measurements, blood samples were obtained in the morning after 12 hours of fasting and subsequently analyzed at each of the three medical centers. FPG levels were measured with a hexokinase method using a Glucose HK Gen.3 kit (Roche Diagnostics, Basel, Switzerland) on a Cobas Integra 800 analyzer (Roche Diagnostics) at AMC, with a hexokinase method using a GLU kit (Roche Diagnostics) on a Roche Modular Analytics system (Roche Diagnostics) at SMC, and with a glucose oxidase method on a TBA 200FR analyzer (Toshiba Medical Systems, Tokyo, Japan) at KUMC. HbA1c levels were measured with a turbidometric inhibition immunoassay (TINIA) using an HbA1c Gen.2 kit (Roche Diagnostics) on a Cobas Integra 800 analyzer (Roche Diagnostics) at AMC, with an high-erformance liquid chromatographic (HPLC) assay using G8 Elution buffers HSi No. 1, No. 2, and No. 3 kits (TOSOH, Yokkaichi, Japan) on a HLC-723G8 (TOSOH) at SMC, and with HPLC on an HA-8180 analyzer (ARKRAY Inc., Kyoto, Japan) at KUMC.
For hormonal measurements, plasma ACTH levels were measured using an immunoradiometric assay (IRMA) using an ELSA-ACTH kit (Cisbio Bioassay, Codolet, France) on a Cobra II Gamma Counter (Packard Instrument Company, Meriden, CT, USA) at AMC, on a RALS Gamma Counter GAMMA-10 (Shin Jin Medics Inc., Seoul, Korea) at SMC, and on a Wallac Wizard 1470 Gamma Counter (Perkin Elmer, Waltham, MA, USA) at KUMC. Serum cortisol and 24-h UFC levels (after dichloromethane extraction) were measured with a radioimmunoassay (RIA) using a Coat-A-Count® Cortisol kit (Siemens Healthcare Diagnostics, Los Angeles, CA, USA) on a Cobra II Gamma Counter (Packard Instrument Company) at AMC, with a RIA using a Cortisol RIA kit (Beckman Coulter, Prague, Czech Republic) on a RALS Gamma Counter GAMMA-10 counter (Shin Jin Medics Inc.) at SMC, and with a RIA using a CORTCT2 kit (Cisbio Bioassay) on a Wallac Wizard 1470 Gamma Counter (Perkin Elmer) at KUMC. Urine concentrations of catecholamine (epinephrine and norepinephrine) were measured with an HPLC assay using a laboratory developed test on an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) at AMC, with an HPLC assay using commercially available HPLC kits (Bio-Rad Laboratories, Hercules, CA, USA) on an Agilent 1200 HPLC system (Agilent Technologies) at SMC, and with an HPLC assay on an Agilent 1260 Infinity system (Agilent Technologies) at KUMC. Urine concentrations of VMA were measured with an HPLC assay using a commercial kit (Bio-Rad Laboratories) on a Flexar HPLC system (Perkin Elmer) at AMC, with an HPLC assay using a commercially available HPLC kit (Bio-Rad Laboratories) on an Agilent 1200 HPLC system (Agilent Technologies) at SMC, and with liquid chromatography-tandem mass spectrometry (LC-MS/MS) on an Agilent 1260 Infinity system (Agilent Technologies) at KUMC. 24-h fractionated metanephrine levels were measured with an HPLC assay using a commercially available kit (Chromsystems, Munich, Germany) on an Agilent 1100 HPLC system (Agilent Technologies) at AMC, with an HPLC assay using an HPLC kit (Bio-Rad Laboratories) on an Agilent 1200 HPLC system (Agilent Technologies) at SMC, and with an HPLC assay on an Agilent 1260 Infinity system (Agilent Technologies) at KUMC. 24-h urinary creatinine levels were measured with a Jaffe kinetic reaction assay using a CREJ kit (Roche Diagnostics) on a Cobas 6000 analyzer (Roche Diagnostics) at AMC, with a Jaffe kinetic reaction assay using a CREJ2 kit (Roche Diagnostics) on a Cobas Integra 800 analyzer (Roche Diagnostics) at SMC, and with an enzymatic method on a TBA 200FR analyzer (Toshiba Medical Systems) at KUMC. DHEA-S levels were measured with a RIA using a Coat-A-Count® DHEA-SO4 kit (Siemens Healthcare Diagnostics) on a Cobra II Gamma Counter (Packard Instrument) at AMC, with RIA using a DHEA-S RIA CT kit (Asbach Medical Products, Obrigheim, Germany) on a RALS Gamma Counter GAMMA-10 (Shin Jin Medics Inc.) at SMC, and with a chemiluminescence immunoassay on an ADVIA Centaur system (Siemens Healthcare Diagnostics) at KUMC. Plasma aldosterone levels and PRA were measured with a RIA using SPAC-S Aldosterone and PRA kits (TFB Inc., Tokyo, Japan), respectively, on a Cobra II Gamma Counter (Packard Instrument Company) at AMC, with a RIA using Aldosterone RIA and Angiotensin I RIA kits (Beckman Coulter) on a RALS Gamma Counter GAMMA-10 (Shin Jin Medics Inc.) at SMC, and with an RIA on a SR300 Gamma Counter (STRATEC, Birkenfeld, Germany) for plasma aldosterone levels and on a Gamma-10 Gamma Counter (Shin Jin Medics Inc.) for PRA at KUMC. The intra- and inter-assay coefficients of variation for all assays were ~5% and 10%, respectively.
Statistical analysis
Continuous and categorical variables are expressed as median values with ranges and numbers with percentages, respectively. To compare continuous variables between the COAR and SIE cohorts, Student's t-test assuming non-equal variance was conducted using median values with ranges. To compare categorical variables between the COAR cohort and the SIE cohort, chi-square or Fisher's exact tests were conducted using numbers with percentages. Clinical characteristics, including age and lesion size, according to histological findings were compared using one-way analysis of variances with Bonferroni adjustment for multiple comparisons. To evaluate the diagnostic values of hypothalamic-pituitary-adrenal (HPA) axis tests for identifying SH in the COAR cohort, the standard methods for calculating sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and accuracy were used. The operating characteristics of the HPA axis tests between the COAR and SIE cohort were compared with a chisquare test using numbers with percentages according to the test results and the diagnosis. We performed multiple logistic regression analysis to generate odds ratios (ORs) and 95% confidence intervals (CIs) for age, sex, and size of masses predicting adrenocortical carcinoma (ACC). The diagnostic values of different diameter cutoffs for identifying ACCs in the COAR cohort were also assessed by calculating sensitivity, specificity, PPV, NPV, and accuracy. Finally, we plotted sensitivity against 1-specificity at each diameter to construct receiver operating characteristics (ROC) curves. The ability to use diameter for predicting ACC was quantified using area under the ROC curve (AUC) analysis. Values of p<0.05 were considered statistically significant, and all statistical analyses were carried out using SAS® ver. 9.1 (SAS Institute, Cary, NC, USA) and SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
All patients included in this study provided written informed consent. All study protocols were approved by the ethics review committee of each institute (IRB numbers: AMC, 2011-0517; SMC, 2011-03-034; KUMC, KUH1010269).
RESULTS
Clinical characteristics of AI patients
The overall numbers of patients eligible for the study were 1005 and 1004 in the COAR and SIE cohorts, respectively. While AIs in the SIE cohort were more frequent in women, our cohort included more men (p<0.001) (Table 1). The median ages were in the 50s in both cohorts, although patients in the COAR cohort were slightly younger (p<0.001). The prevalence of comorbidities (e.g., hypertension, diabetes, and obesity) was higher in the COAR cohort than in the SIE cohort (all p<0.001) (Table 1). In the COAR cohort, the prevalence of hypertension and obesity was significantly different between different functional classifications of AIs (all p<0.001), while no difference was observed in the prevalence of diabetes. The prevalence of lower bone mass was marginally different between different functional classifications of AIs (p=0.091) (Supplementary Table 1, only online).
Table 1. Clinical Characteristics of Patients with Adrenal Incidentalomas Included in the COAR and SIE Cohorts.
| Variables | COAR (n=1005) | SIE (n=1004) | p value† |
|---|---|---|---|
| Sex | <0.001 | ||
| Men | 579 (57.6) | 420 (42.0) | |
| Women | 426 (42.4) | 584 (58.0) | |
| Age (yr) | 55.0 (20.0–80.0) | 58.0 (15.0–86.0) | <0.001 |
| Comorbidity | |||
| Hypertension | 518 (51.5) | 362 of 884 (41.0)* | <0.001 |
| Diabetes | 248 (24.7) | 87 of 873 (10.0)* | <0.001 |
| Obesity | 532 (53.1) | 233 of 823 (28.0)* | <0.001 |
| Site of mass | <0.001 | ||
| Right | 308 (30.6) | 596 (59.0) | |
| Left | 594 (59.1) | 307 (31.0) | |
| Bilateral | 103 (10.2) | 101 (10.0) | |
| Diameter of mass (cm) | 1.7 (1.0–17.0) | 3.0 (0.5–25.0) | <0.001 |
| Right | 1.8 (1.0–17.0) | 2.8 (1.0–10.0) | <0.001 |
| Left | 1.6 (1.0–12.2) | 2.5 (1.0–13.0) | <0.001 |
| Mass less than 4 cm | 945 (94.0) | 158 of 247 (64.0)* | <0.001 |
| Operation | 195 (19.4) | 380 (37.8) | <0.001 |
| Age at operation (yr) | 51.0 (20.0–76.0) | 55.0 (15.0–84.0) | <0.001 |
| Size at operation (cm) | 2.5 (1.0–17.0) | 4.0 (1.0–25.0) | NA |
| Histological pictures | <0.001 | ||
| Adenoma | 116 (59.5) | 198 (52.0) | <0.001 |
| Carcinoma | 10 (5.1) | 47 (12.0) | <0.001 |
| Cyst | 1 (0.5) | 20 (5.0) | <0.001 |
| Myelolipoma | 6 (3.1) | 30 (8.0) | <0.001 |
| Metastasis | 3 (1.5) | 7 (2.0) | 0.225 |
| Ganglioneuroma | 3 (1.5) | 15 (4.0) | 0.009 |
| Pheochromocytoma | 53 (27.2) | 42 (11.0) | 0.295 |
| Others | 3 (1.5) | 21 (6.0) | <0.001 |
| Functional classification | <0.001 | ||
| Nonfunctional adrenal mass | 837 (83.3) | 854 (85.0) | 0.303 |
| Subclinical hypercortisolism | 44 (4.4) | 92 (9.2) | <0.001 |
| Pheochromocytoma | 60 (6.0) | 42 (4.2) | 0.084 |
| Primary aldosteronism | 61 (6.1) | 16 (1.6) | <0.001 |
| Others | 3 (0.3) | 0 (0.0) | 0.250 |
COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; NA, not applicable.
Data of the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 Continuous variables are shown as median values with ranges and categorical variables are shown as numbers with percentages.
*Indicates the total number of patients that were available for analysis, †p values were generated with Student's t-test assuming non-equal variance for continuous variables and with chi-square or Fisher's exact tests for categorical variables.
Significantly fewer and younger patients underwent adrenalectomy in the COAR cohort than in the SIE cohort (both p<0.001) (Table 1). In both cohorts, patients who underwent adrenalectomy were significantly younger than those who did not (51.0 years vs. 56.0 years; p<0.001 in the COAR cohort, and 55.0 years vs. 60.0 years; p<0.001 in the SIE cohort). Surgically removed masses were mostly adrenal cortical adenomas in both cohorts. Histologically, ACCs, cysts, myelolipomas, ganglioneuromas, and other histological diagnoses were more prevalent in the SIE cohort than in the COAR cohort (all p<0.001), whereas the prevalence of metastases and pheochromocytomas was comparable in both cohorts (Table 1). Despite the smaller number of women than men in the COAR cohort, more women underwent surgeries due to histologic findings (e.g., adrenal cortical adenomas, ACCs, and pheochromocytomas) (Table 2). In the COAR cohort, median ages were not significantly different according to histologic findings, while in the SIE cohort, patients with ACCs were younger than those with adrenal cortical adenomas (p<0.001) (Table 2).
Table 2. Characteristics of Adrenal Incidentalomas According to Histology in the COAR and SIE Cohorts.
| Variables | COAR | SIE | p value* |
|---|---|---|---|
| Cortical adenoma | |||
| Sex | 0.760 | ||
| Men | 55 (47.4) | 89 (45.0) | |
| Women | 61 (52.6) | 109 (55.0) | |
| Age (yr) | 53.0 (27.0–74.0) | 57.0 (16.0–83.0) | <0.001 |
| Diameter (cm) | 2.0 (1.0–5.3) | 3.5 (1.0–15.0) | NA |
| Adrenocortical carcinoma | |||
| Sex | 0.371 | ||
| Men | 3 (30.0) | 23 (49.0) | |
| Women | 7 (70.0) | 24 (51.0) | |
| Age (yr) | 49.5 (20.0–62.0) | 46.0 (17.0–84.0) | 0.406 |
| Diameter (cm) | 4.4 (1.5–13.0) | 7.5 (2.6–25.0) | NA |
| Cyst | |||
| Sex | 0.286 | ||
| Men | 1 (100.0) | 5 (25.0) | |
| Women | 0 (0.0) | 15 (75.0) | |
| Age (yr) | 45.0 (45.0–45.0) | 47.0 (18.0–67.0) | 0.875 |
| Diameter (cm) | 6.8 (6.8–6.8) | 4.5 (2.8–18.0) | NA |
| Myelolipoma | |||
| Sex | 0.020 | ||
| Men | 6 (100.0) | 13 (43.0) | |
| Women | 0 (0.0) | 17 (57.0) | |
| Age (yr) | 48.5 (32.0–59.0) | 52.0 (26.0–72.0) | 0.333 |
| Diameter (cm) | 7.1 (4.3–12.7) | 5.0 (2.5–12.0) | NA |
| Metastasis | |||
| Sex | 1.000 | ||
| Men | 3 (100.0) | 5 (71.0) | |
| Women | 0 (0.0) | 2 (29.0) | |
| Age (yr) | 64.0 (47.0–70.0) | 58.0 (46.0–70.0) | 0.210 |
| Diameter (cm) | 2.0 (1.5–7.2) | 6.4 (3.5–12.0) | NA |
| Ganglioneuroma | |||
| Sex | 0.528 | ||
| Men | 2 (66.7) | 5 (33.0) | |
| Women | 1 (33.3) | 10 (67.0) | |
| Age (yr) | 22.0 (20.0–65.0) | 45.0 (16.0–76.0) | 0.049 |
| Diameter (cm) | 5.1 (4.7–5.2) | 5.0 (2.6–11.5) | NA |
| Pheochromocytoma | |||
| Sex | 0.664 | ||
| Men | 23 (43.4) | 21 (49.0) | |
| Women | 30 (56.6) | 21 (51.0) | |
| Age (yr) | 48.0 (23.0–76.0) | 54.0 (26.0–79.0) | 0.031 |
| Diameter (cm) | 3.8 (1.0–17.0) | 5.0 (2.1–10.0) | NA |
| Other | |||
| Sex | 1.000 | ||
| Men | 1 (33.3) | 6 (27.0) | |
| Women | 2 (66.7) | 15 (73.0) | |
| Age (yr) | 54.0 (39.0–56.0) | 60.0 (15.0–77.0) | 0.176 |
| Diameter (cm) | 3.8 (1.3–4.9) | 4.2 (1.7–11.0) | NA |
COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; NA, not applicable.
Data of the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 Continuous variables are shown as median values with ranges, and categorical variables are shown as numbers with percentages.
*p values were generated using Student's t-test assuming non-equal variance for continuous variables and using chi-square or Fisher's exact tests for categorical variables. Post-hoc analyses in the COAR cohort: Age, no significant relations. Size, cortical adenoma vs. cortical carcinoma, p<0.001; cortical adenoma vs. myelolipoma, p<0.001; cortical adenoma vs. pheochromocytoma, p<0.001; myelolipoma vs. pheochromocytoma, p=0.002; myelolipoma vs. other, p=0.026. Cysts were excluded from the post-hoc analyses in the COAR cohort. Post-hoc analyses in the SIE cohort: Age, cortical adenoma vs. cortical carcinoma, p=0.050; cortical adenoma vs. cyst, p<0.050; cortical adenoma vs. ganglioneuroma, p=0.037; cyst vs. pheochromocytoma, p=0.038; ganglioneuroma vs. pheochromocytoma, p=0.020. Size, cortical adenoma vs. adrenocortical carcinoma, p<0.001; adrenocortical carcinoma vs. pheochromocytoma, p<0.001.
Functional classifications of AIs
According to the hormonal evaluations, AIs in the COAR cohort were mostly nonfunctional (83.3%), and the remainders were SH (4.4%), pheochromocytomas (6.0%), and PA (6.1%). Compared with the SIE cohort, SH cases were fewer (p<0.001) and PA was more common (p<0.001) in the COAR cohort (Table 1). Among 61 patients with PA in the COAR cohort, nine (14.7%) patients also showed SH.
In 837 patients with nonfunctional AIs in the COAR cohort, the following single alterations in the HPA axis were observed: low ACTH levels in 67 patients (8.0%), high UFC levels in 74 patients (8.8%), lack of suppression in 1 mg-DST in 42 patients (5.0%), and low DHEA-S levels in 309 patients (36.9%) (Supplementary Fig. 1, only online). Among 44 SH patients, 36 (81.8%), 24 (54.5%), 32 (72.7%), and 22 (50.0%) patients showed low ACTH levels, high UFC levels, lack of suppression in 1 mg-DST, and low DHEA-S levels, respectively. While comparable numbers of SH patients in both COAR and SIE cohorts exhibited abnormalities in ACTH levels and 1 mg-DST, fewer nonfunctional AIs exhibiting abnormalities were observed in the COAR cohort (all p<0.001) (Supplementary Fig. 1, only online). For UFC levels, fewer SH patients in the COAR cohort showed abnormally high values (p=0.028) (Supplementary Fig. 1, only online). In both cohorts, the two most frequent hormonal abnormalities simultaneously observed in SH patients were lack of suppression in 1 mg-DST and low ACTH levels (54.5% and 55.5% in the COAR and SIE cohorts, respectively; p=1.000). Fewer SH patients exhibiting both a lack of suppression in 1 mg-DST and high UFC levels were observed in the COAR cohort than in the SIE cohort (25.0% vs. 50.0%; p=0.018).
The operating characteristics of hormonal tests for diagnosing SH are shown in Table 3. While the specificity, NPV, and accuracy of the ACTH test (p<0.001, p=0.002, and p<0.001, respectively) and 1 mg-;DST (all p<0.001) in the COAR cohort were higher than those in the SIE cohort, the sensitivity and PPV of the UFC test (p=0.028 and p<0.001, respectively) in the COAR cohort were lower.
Table 3. Operating Characteristics of Hypothalamic-Pituitary-Adrenal Axis tests to Qualify Patients for Subclinical Hypercortisolism in the COAR and SIE Cohorts.
| Tests | COAR | SIE | p value* |
|---|---|---|---|
| Low ACTH (%) | |||
| Sensitivity | 81.8 | 79.0 | 0.941 |
| Specificity | 92.0 | 85.0 | <0.001 |
| PPV | 35.0 | 47.0 | 0.076 |
| NPV | 99.0 | 96.0 | 0.002 |
| Accuracy | 91.5 | 84.1 | <0.001 |
| High UFC (%) | |||
| Sensitivity | 54.5 | 76.0 | 0.028 |
| Specificity | 91.2 | 88.0 | 0.129 |
| PPV | 24.5 | 49.0 | <0.001 |
| NPV | 97.4 | 96.0 | 0.238 |
| Accuracy | 89.3 | 86.8 | 0.165 |
| Positive 1 mg-DST (%) | |||
| Sensitivity | 72.7 | 73.0 | 1.000 |
| Specificity | 95.0 | 90.0 | <0.001 |
| PPV | 43.2 | 57.0 | 0.094 |
| NPV | 98.5 | 95.0 | <0.001 |
| Accuracy | 93.9 | 87.5 | <0.001 |
COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; ACTH, adrenocorticotropic hormone level; UFC, 24-h urinary free cortisol excretion; 1 mg-DST, 1 mg overnight dexamethasone suppression test; PPV, positive predictive value; NPV, negative predictive value.
Data of the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8
*p values were generated using the chi-square test.
Among 60 patients with pheochromocytoma in the COAR cohort, 40 (66.7%) had high urinary catecholamine levels, 27 (45.0%) had high urinary VMA levels, and 51 (85.0%) had high urinary fractionated metanephrine levels (Supplementary Fig. 2, only online). In both cohorts, a similar number of patients had abnormal biochemical data, and about half of the patients with pheochromocytomas was hypertensive (Supplementary Fig. 2, only online). Among 60 patients with pheochromocytoma who provided the presence of typical symptoms, 15 (25.0%), 14 (23.3%), and six (10.0%) patients had complained of headache, palpitation, and sweating, respectively, at their initial visits.
Among PA patients, fewer patients in the COAR cohort (63.9% vs. 100.0%; p=0.004) showed suppression in PRA, whereas more patients showed elevated plasma aldosterone levels (93.4% vs. 69.0%; p=0.028) (Supplementary Fig. 3, only online).
Characteristics of AIs
All AIs in the COAR cohort were detected using abdominal or chest CT, while only 28.0% of AIs were initially discovered on abdominal CT in the SIE cohort.8 In the COAR cohort, AIs were more frequent on the left side, whereas they were more frequent on the right side in the SIE cohort (p<0.001) (Table 1). The frequency of bilateral masses was about 10.0% in both cohorts. In the COAR cohort, masses were mostly <4 cm, and the size was not significantly different according to the site (p=0.298). However, masses in the COAR cohort were significantly smaller than those in the SIE cohort (p<0.001), differences that were maintained even after evaluation by site (all p<0.001) (Table 1).
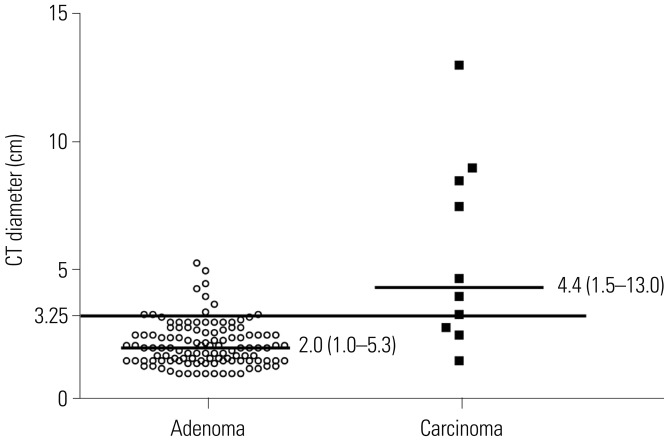
In both cohorts, ACCs were significantly larger than those confirmed as adenomas (all p<0.001) (Table 2, Fig. 1). In the COAR cohort, logistic regression analyses of age, sex, and mass size as predictors of the risk of ACCs showed that only mass size was correlated with an increased risk of malignancy (OR, 1.32; 95% CI, 1.09-1.59). As shown in Supplementary Table 2 (only online), a mass size for identifying ACCs of around 3 cm to 4 cm had both high sensitivity and specificity in the COAR cohort, compared to 5 cm to 6 cm in the SIE cohort.8 Furthermore, the best cutoff value for size in the ROC curve, corresponding to Youden's index,25 was 3.25 cm (sensitivity: 70.0% and specificity: 69.7%) in the COAR cohort and 5.0 cm in the SIE cohort.8 The AUCs were 0.75 (p=0.007) in the COAR cohort and 0.84 (p<0.001) in the SIE cohort.8 The scattered plots in the COAR cohort also showed that the cutoff value of 3.25 cm obtained from ROC curves quite clearly classified adrenal cortical adenomas from carcinomas, even though there were some overlap (Figure 1).
Fig. 1. Comparison of mass sizes of histologically proven adrenal cortical adenomas and carcinomas in the COAR cohort. Median values with ranges are shown. The cutoff value of 3.25 cm was determined according to the best cutoff values in receiver operating characteristics curves, corresponding to Youden's index.25 COAR, Co-work of Adrenal Research; CT, computed tomography.
DISCUSSION
To our knowledge, this is the first and the largest study in Asians that prospectively recruited more than 1000 AI patients based on CT examinations. We not only investigated the characteristics of AIs in Korean patients (the COAR cohort), but also compared them to those reported in a previous large-scale study on Italian patients (the SIE cohort). The results showed some inconsistencies between the patient cohorts. AIs in Korean patients were more frequent in men and predominantly observed on the left side. Patients with AIs were slightly younger, and fewer AIs were surgically removed in the Korean study. Most AIs were nonfunctional in both studies, while fewer SH and more PA cases were detected in the Korean study. In Korean patients, hormonal abnormalities (e.g., low ACTH levels and positive 1 mg-DST results), more specifically diagnosed SH, and high UFC levels showed very low sensitivity compared to those in Italian patients. In pheochromocytoma or PA, there were no notable hormonal differences between studies. AIs were smaller in Korean patients than in Italian patients; consistently, a lower cutoff size for distinguishing ACCs from benign masses might be proposed in Korean patients.
The biggest difference between these studies lies in their timeframes: the subjects of the Italian study were recruited from 1980 to 1995, while those of our study were recruited from 2011 to 2014. Since the SIE study, there have been great advances in the techniques for evaluating AIs, especially in the resolution of CT. While earlier CT studies reported a prevalence of AIs up to 0.9%,26 recent studies have reported incidences of 4.0-5.0%.27,28 Moreover, the development of new CT protocols has improved the detection and characterization of adrenal masses.29,30 In this perspective, the distinctive characteristics of AIs in the Korean study (e.g., left side predominance and smaller size) possibly indicate the true features of AIs that were not reliably detectable prior to the improvement of radiologic techniques. The smaller numbers of Korean patients undergoing adrenalectomy might originate from the prevention of unnecessary surgery by the detailed characterization of AIs using improved imaging techniques. In contrast, there is also a possibility that the differences in the characteristics of AIs between the Korean and Italian studies were due to racial differences. However, previous studies using mainly US imaging (>70 %) in Koreans,31 Chinese,11 and Italians8 showed right side predominance and larger-sized AIs (3.0–5.6 cm), whereas those using mainly CT imaging (>70%) in Koreans12,16 and Japanese32 showed left side predominance and smaller size (1.7–3.0 cm), regardless of the study population. Consistently, US imaging detected right-sided AIs more often than left-sided ones.29,33,34 Therefore, race may not contribute to the clinical aspects of AIs. The male predominance of AIs, which was also observed in other Korean studies,16,31 and younger age of this population may reflect the pattern of health care use in Korea.
In this study, we observed fewer SH and more PA cases than reported previously.1,2,8,35 We used a 1 mg-DST cutoff of 5.0 µg/dL for diagnosing SH, which was also used in the Italian study, but is still a matter of debate.33 In previous studies, a 1 mg-DST cutoff of 5.0 µg/dL showed good specificity (83.0-100.0%) with low sensitivity (44.0-58.0%), while a cutoff of 50.0 nmol/L showed good sensitivity (75.0–100.0%) with low specificity (44.0–58.0%).33 Therefore, the observation of fewer SH cases in this study suggests that mildly abnormal cortisol secretion may be missed by a high 1 mg-DST cutoff in this population with AIs of small size. Consistently, studies with high cutoffs for 1 mg-DST values32 showed fewer SH and more PA cases than those with lower cutoff values.16 These suggest that a lower 1 mg-DST cutoff might be needed for diagnosing SH. Even subclinical PA, which may reflect the early stages of overt disease when small tumors mildly secrete hormones, can cause deleterious consequences.34 It is also known that aldosterone excess itself has an adverse effect on cardiac function in normotensive patients with PA.36 Therefore, proper diagnosis and active management of this disease are needed. Since most subclinical PAs are discovered as AIs, some groups recommended screening for PA in all AI patients,34 although the latest guideline released by the US Endocrine Society recommend screening only in hypertensive AI patients.37 In our study, we screened for PA in all patients. Therefore, the increased diagnosis of PA might be attributed to the detection of small tumors that mildly secrete hormones. Consistently, among PA patients, most showed a mild increase in systolic blood pressure below <160 mm Hg [n=50 (83.3%)], and few were even normotensive [n=3 (4.9%)].
Although much time has passed since the concept of SH was initially introduced, there is no consensus on the clinical and/ or biochemical criteria with which to diagnose this condition that eventually predict a benefit of surgery.38 To date, many authors proposed a combination of various HPA axis parameters (e.g., cortisol levels after 1 mg-DST, altered circadian cortisol rhythm, low ACTH levels, low DHEA-S levels, or UFC levels) for diagnosing SH.33 However, our study showed that, while ACTH levels and 1 mg-DST values showed higher specificity and accuracy than those in the Italian population, UFC levels showed notably poorer performance. UFC cannot reliably reveal a slight cortisol alteration and has technical problems associated with its determination.33 Therefore, UFC should not be considered an adequate screening test for SH and should be used in combination with other tests.33
ACCs are rare, but they are one of the most aggressive endocrine malignancies detected incidentally in ~15% of cases.39 ACCs are suggested by a large diameter (>6 cm), irregular shape, heterogeneous histology, and calcification.6 Recent studies demonstrated that a cutoff of 4 cm in size and a CT attenuation value of ≤10 Hounsfield units has great accuracy in distinguishing benign from malignant tumors.2,7 However, the best cutoff value for mass size in the COAR cohort for predicting ACC was 3.25 cm and was lower than that in the SIE cohort.8 Although the reason is currently uncertain, except for the advance in CT technology detecting smaller AIs, a recent retrospective study investigating Korean AIs also suggested an optimal cutoff value for mass size as 3.4 cm for differentiating malignant from benign lesions,40 similar with the cutoff value in the COAR cohort. Given the high mortality rate associated with ACCs, we suggest excision of lesions ≥3 cm.
This is the first and the largest prospective multi-center study on AIs in an Asian population using CT scans. Compared with previous studies in mainly Western populations, which had some limitations (e.g., retrospective design, small patient cohorts, or suboptimal imaging techniques), our study provides more accurate information about the characteristics of AIs, which can aid their effective management.
However, our comparative study was limited in that we could not obtain detailed data from the SIE cohort. Especially, the indications for operation of AIs were not presented in the SIE cohort although the different indications for operation between studies could result in the difference in the baseline characteristics of AIs. There were also some differences in the definition of diseases between the Korean and Italian studies. Additionally, the number of ACC patients in the COAR cohort was too few to assure the cutoff in size between benign and malignant masses. Lastly, it would be also meaningful if we could compare the baseline characteristics of Korean AI patients in two different timeframes. However, no Korean study has investigated as large of numbers of AI patients as ours so far.
In summary, recent advances in CT technology could be used to accurately characterize AIs. The necessity for surgery may also be reduced for smaller AIs when malignancy is suspected. Based on the baseline characteristics of AIs in this prospective COAR study, more meaningful follow-up studies should be conducted in the near future.
ACKNOWLEDGEMENTS
This study was supported by grants from the Asan Institute for Life Sciences, Seoul, Republic of Korea (Project No. 2014-1215) and Dong-A ST (Seoul, Korea).
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIALS
The proportion of hypothalamic-pituitary-adrenal axis abnormalities in patients with SH relative to non-functional adrenal masses in the COAR and SIE cohort. To compare the numbers of patients between the COAR and SIE cohorts, chi-square test or Fisher's exact tests were conducted. Data from the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 *Nonfunctional adrenal mass in COAR vs. SIE, p<0.05, †SH in COAR vs. SIE, p<0.05. COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; SH, Subclinical Hypercortisolism; ACTH, adrenocorticotrophic hormone; UFC, 24-h urinary free cortisol; 1 mg-DST, 1 mg-dexamethasone suppression test; DHEA-S, dehydroepiandrosterone-sulfate.
Biochemical and clinical characteristics of patients with pheochromocytomas in the COAR and SIE cohorts. To compare the numbers of patients who showed positive hormonal results or had hypertension between the COAR and SIE cohorts, a chi-square test was conducted. Data for the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; VMA, vanillylmandelic acid; U, 24-h urine; P, plasma; NA, not applicable.
Biochemical and clinical characteristics of patients with primary aldosteronism in the COAR and SIE cohorts. To compare the numbers of patients who showed positive hormonal results or had hypertension between the COAR and SIE cohorts, a Fisher's exact test was conducted. Data for the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 *p<0.05. COAR, Co-work of Adrenal Research; SIE, Italian Society of Endocrinology; PRA, plasma renin activity; ARR, aldosterone-to-renin ratio.
Prevalence of Comorbidities according to the Functional Classifications of Adrenal Incidentaloma in the COAR Cohort
Diagnostic Power of Different Cutoff Values for Mass Size for the Differentiation of Adrenal Cortical Carcinomas from Benign Masses in the COAR and SIE Cohorts
References
- 1.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–484. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 2.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164:851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 3.Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26:405–419. doi: 10.1016/j.beem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosi B, Peverelli S, Passini E, Re T, Ferrario R, Colombo P, et al. Abnormalities of endocrine function in patients with clinically "silent" adrenal masses. Eur J Endocrinol. 1995;132:422–428. doi: 10.1530/eje.0.1320422. [DOI] [PubMed] [Google Scholar]
- 5.Androulakis II, Kaltsas G, Piaditis G, Grossman AB. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41:552–560. doi: 10.1111/j.1365-2362.2010.02436.x. [DOI] [PubMed] [Google Scholar]
- 6.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95:4106–4113. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 8.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 9.Kasperlik-Załuska AA, Otto M, Cichocki A, Rosłonowska E, Słowin´ J, Jeske W, et al. Incidentally discovered adrenal tumors: a lesson from observation of 1,444 patients. Horm Metab Res. 2008;40:338–341. doi: 10.1055/s-2008-1073167. [DOI] [PubMed] [Google Scholar]
- 10.Bhargav PR, Mishra A, Agarwal G, Agarwal A, Verma AK, Mishra SK. Adrenal incidentalomas: experience in a developing country. World J Surg. 2008;32:1802–1808. doi: 10.1007/s00268-008-9550-8. [DOI] [PubMed] [Google Scholar]
- 11.Bin X, Qing Y, Linhui W, Li G, Yinghao S. Adrenal incidentalomas: experience from a retrospective study in a Chinese population. Urol Oncol. 2011;29:270–274. doi: 10.1016/j.urolonc.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Bae KH, Choi YK, Jeong JY, Park KG, Kim JG, et al. Clinical characteristics for 348 patients with adrenal incidentaloma. Endocrinol Metab (Seoul) 2013;28:20–25. doi: 10.3803/EnM.2013.28.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comlekci A, Yener S, Ertilav S, Secil M, Akinci B, Demir T, et al. Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single centre experience. Endocrine. 2010;37:40–46. doi: 10.1007/s12020-009-9260-5. [DOI] [PubMed] [Google Scholar]
- 14.Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99:827–834. doi: 10.1210/jc.2013-3527. [DOI] [PubMed] [Google Scholar]
- 15.Yeomans H, Calissendorff J, Volpe C, Falhammar H, Mannheimer B. Limited value of long-term biochemical follow-up in patients with adrenal incidentalomas-a retrospective cohort study. BMC Endocr Disord. 2015;15:6. doi: 10.1186/s12902-015-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YY, Suh S, Joung JY, Jeong H, Je D, Yoo H, et al. Clinical characteristics and follow-up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013;28:557–564. doi: 10.3904/kjim.2013.28.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu J, Festen EA, Wijmenga C. Multi-ethnic studies in complex traits. Hum Mol Genet. 2011;20:R206–R213. doi: 10.1093/hmg/ddr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morelli V, Donadio F, Eller-Vainicher C, Cirello V, Olgiati L, Savoca C, et al. Role of glucocorticoid receptor polymorphism in adrenal incidentalomas. Eur J Clin Invest. 2010;40:803–811. doi: 10.1111/j.1365-2362.2010.02330.x. [DOI] [PubMed] [Google Scholar]
- 19.Siggelkow H, Etmanski M, Bozkurt S, Groβ P, Koepp R, Brockmoller J, et al. Genetic polymorphisms in 11β-hydroxysteroid dehydrogenase type 1 correlate with the postdexamethasone cortisol levels and bone mineral density in patients evaluated for osteoporosis. J Clin Endocrinol Metab. 2014;99:E293–E302. doi: 10.1210/jc.2013-1418. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Sasagawa I, Suzuki H, Izumi T, Kaneko H, Nakada T. The role of ultrasonography in the detection of adrenal masses: comparison with computed tomography and magnetic resonance imaging. Int Urol Nephrol. 2001;32:303–306. doi: 10.1023/a:1017583211460. [DOI] [PubMed] [Google Scholar]
- 21.Tabarin A, Bardet S, Bertherat J, Dupas B, Chabre O, Hamoir E, et al. Exploration and management of adrenal incidentalomas. French Society of Endocrinology Consensus. Ann Endocrinol (Paris) 2008;69:487–500. doi: 10.1016/j.ando.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 24.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Young WF., Jr Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000;29:159–185. doi: 10.1016/s0889-8529(05)70122-5. [DOI] [PubMed] [Google Scholar]
- 27.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol. 2002;179:559–568. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 28.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190:1163–1168. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 29.Blake MA, Kalra MK, Sweeney AT, Lucey BC, Maher MM, Sahani DV, et al. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology. 2006;238:578–585. doi: 10.1148/radiol.2382041514. [DOI] [PubMed] [Google Scholar]
- 30.Foti G, Faccioli N, Mantovani W, Malleo G, Manfredi R, Mucelli RP. Incidental adrenal lesions: accuracy of quadriphasic contrast enhanced computed tomography in distinguishing adenomas from nonadenomas. Eur J Radiol. 2012;81:1742–1750. doi: 10.1016/j.ejrad.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Kim HY, Kim SG, Lee KW, Seo JA, Kim NH, Choi KM, et al. Clinical study of adrenal incidentaloma in Korea. Korean J Intern Med. 2005;20:303–309. doi: 10.3904/kjim.2005.20.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabuchi Y, Otsuki M, Kasayama S, Kosugi K, Hashimoto K, Yamamoto T, et al. Clinical and endocrinological characteristics of adrenal incidentaloma in Osaka region, Japan. Endocr J. 2016;63:29–35. doi: 10.1507/endocrj.EJ15-0404. [DOI] [PubMed] [Google Scholar]
- 33.Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96:1223–1236. doi: 10.1210/jc.2010-2722. [DOI] [PubMed] [Google Scholar]
- 34.Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2012;26:485–495. doi: 10.1016/j.beem.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–285. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 36.Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90:5070–5076. doi: 10.1210/jc.2005-0681. [DOI] [PubMed] [Google Scholar]
- 37.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 38.Nieman LK. Update on subclinical Cushing's syndrome. CurrOpin Endocrinol Diabetes Obes. 2015;22:180–184. doi: 10.1097/MED.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 39.Dackiw AP, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World J Surg. 2001;25:914–926. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 40.Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, et al. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrino. 2017;177:475–483. doi: 10.1530/EJE-17-0372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proportion of hypothalamic-pituitary-adrenal axis abnormalities in patients with SH relative to non-functional adrenal masses in the COAR and SIE cohort. To compare the numbers of patients between the COAR and SIE cohorts, chi-square test or Fisher's exact tests were conducted. Data from the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 *Nonfunctional adrenal mass in COAR vs. SIE, p<0.05, †SH in COAR vs. SIE, p<0.05. COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; SH, Subclinical Hypercortisolism; ACTH, adrenocorticotrophic hormone; UFC, 24-h urinary free cortisol; 1 mg-DST, 1 mg-dexamethasone suppression test; DHEA-S, dehydroepiandrosterone-sulfate.
Biochemical and clinical characteristics of patients with pheochromocytomas in the COAR and SIE cohorts. To compare the numbers of patients who showed positive hormonal results or had hypertension between the COAR and SIE cohorts, a chi-square test was conducted. Data for the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 COAR, Co-work Of Adrenal Research; SIE, Italian Society of Endocrinology; VMA, vanillylmandelic acid; U, 24-h urine; P, plasma; NA, not applicable.
Biochemical and clinical characteristics of patients with primary aldosteronism in the COAR and SIE cohorts. To compare the numbers of patients who showed positive hormonal results or had hypertension between the COAR and SIE cohorts, a Fisher's exact test was conducted. Data for the SIE cohort were modified according to those reported by Mantero, et al. J Clin Endocrinol Metab 2000;85:637-44.8 *p<0.05. COAR, Co-work of Adrenal Research; SIE, Italian Society of Endocrinology; PRA, plasma renin activity; ARR, aldosterone-to-renin ratio.
Prevalence of Comorbidities according to the Functional Classifications of Adrenal Incidentaloma in the COAR Cohort
Diagnostic Power of Different Cutoff Values for Mass Size for the Differentiation of Adrenal Cortical Carcinomas from Benign Masses in the COAR and SIE Cohorts