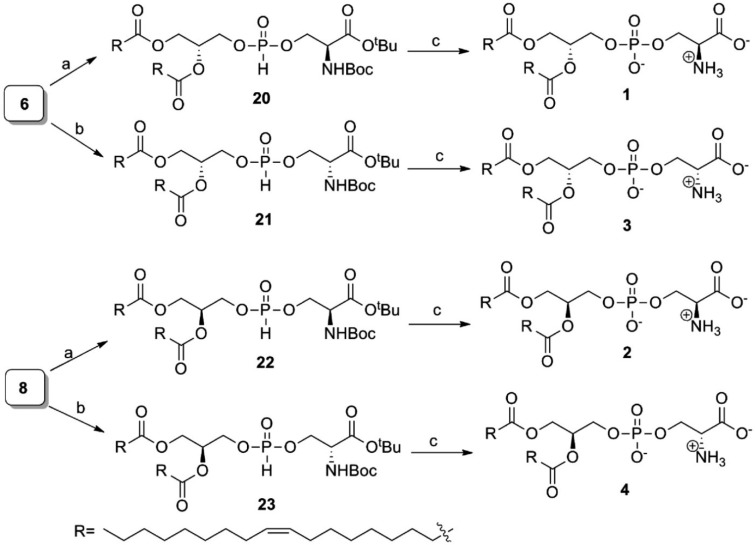
Scheme 3. Syntheses of 1–4.
Reagents and conditions: (a) (S)-tert-butyl 2-((tert-butoxycarbonyl)amino)-3-hydroxypropanoate, PivCl and pyridine for 0.5 h at rt, 90–95%; (b) (R)-tert-butyl 2-(tert-butoxycarbonyl)amino)-3-hydroxypropanoate, PivCl and pyridine for 0.5 h at rt, 90–98%; (c) (i) I2 and 95% pyridine-H2O at rt; (ii) TFA and CH2Cl2 at 0 °C, 98%.