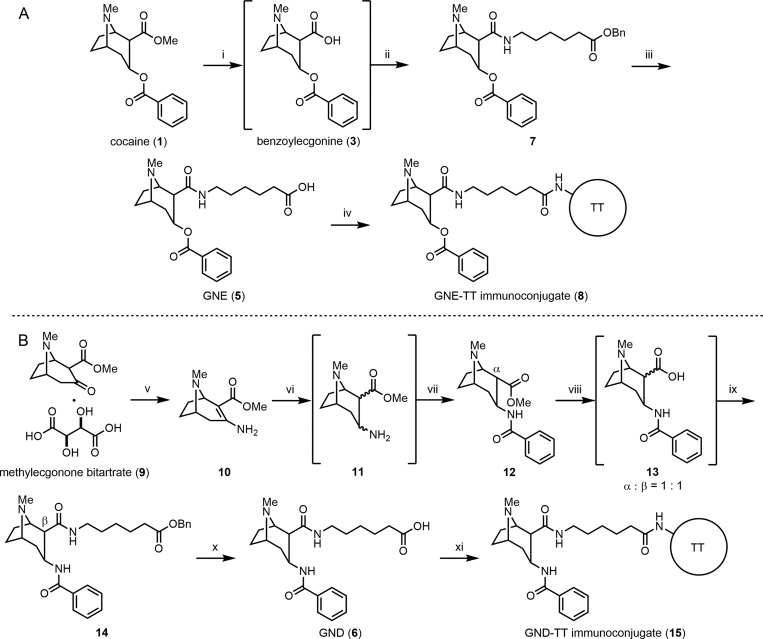
Scheme 1.
Reagents and conditions: (i) H2O, 1,4-dioxane, MW, 40 W, 90 psi, 130 °C, 2 h, then evap.; (ii) benzyl 6-aminohexanoate, DMTMM, Et3N, THF, rt, 52% (one-pot); (iii) H2, Pd/C, EtOH, rt, 68%; (iv) sulfo-NHS, EDCI, DMF/H2O, rt, 12 h, then TT, PBS, 4 °C, 24 h; (v) sat. NH3 in MeOH, MW, 300 W, 150 psi, 100 °C, 4 h, 59%; (vi) NaBH3CN, 2 M HCl in 1,4-dioxane, MeOH, 33 h, then evap.; (vii) BzCl, NaHCO3, 1,4-dioxane/H2O, rt, 20% (one-pot); (viii) H2O, reflux, 12 h, then evap.; (ix) benzyl 6-aminohexanoate toluenesulfonic acid salt, EDCI, Et3N, DMAP, CH2Cl2, 16% (one-pot); (x) H2, Pd/C, EtOH, rt, 94%; (xi) sulfo-NHS, EDCI, DMF/H2O, rt, 12 h, then TT, PBS, 4 °C, 24 h.