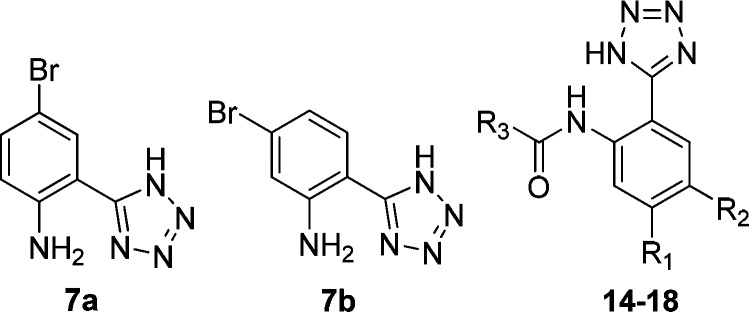
Table 1. Potency of 2-Substituted 2-(1H-Tetrazol-5-yl)aniline Derivatives in DMR Assays.
| compd | R1 | R2 | R3 | EC50a (μM) | desensitization IC50b (μM) | antagonist IC50c (μM) |
|---|---|---|---|---|---|---|
| 7a | 29.99 ± 2.80 | 35.56 ± 2.54 | 0.67 ± 0.19 | |||
| 7b | 44.06 ± 4.06 | 75.34 ± 9.31 | 0.67 ± 0.25 | |||
| 14 | Br | H | ethyl | 0.62 ± 0.06 | 0.70 ± 0.07 | 0.47 ± 0.11 |
| 15 | Br | H | isopropyl | 0.38 ± 0.02 | 0.28 ± 0.02 | 0.58 ± 0.06 |
| 16 | Br | H | cyclohexyl | 0.44 ± 0.03 | 0.20 ± 0.01 | 0.43 ± 0.08 |
| 17 | Br | H | furyl | 1.06 ± 0.13 | 0.44 ± 0.03 | 0.29 ± 0.08 |
| 18 | Br | H | thienyl | 0.51 ± 0.02 | 0.26 ± 0.03 | 0.50 ± 0.05 |
EC50 to trigger DMR.
IC50 to desensitize upon cells repeated stimulation with 1 μM zaprinast.
IC50 of known GPR35 antagonist 5 to block the agonism. The data respresent mean ± sd from two independent measurements, each with four replicates (n = 8).