Scheme 1. Design (a) and Synthesis (b) of 3-Benzyl Coumarin and Phenylsulfonylfuroxan Hybrids and Their Seco-B-ring Derivatives.
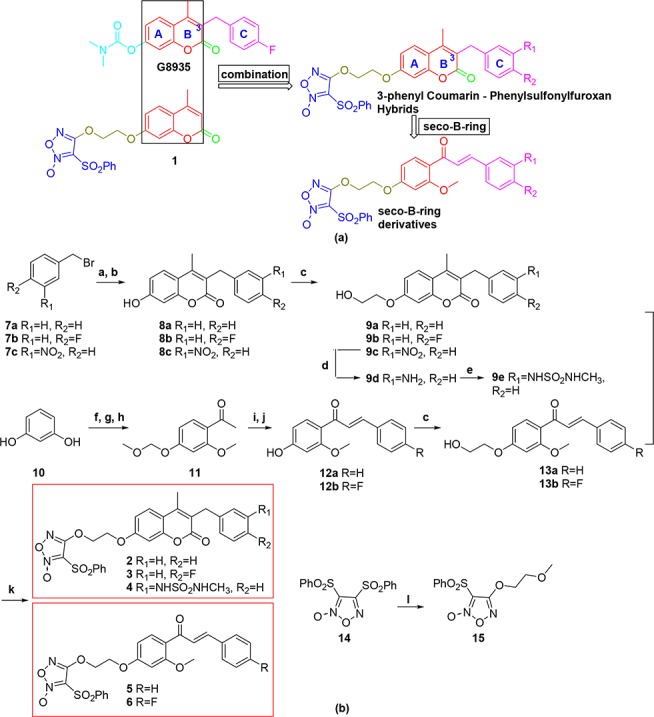
Reagents and conditions: (a) ethyl 3-oxobutanoate (1.0 equiv), NaH (1.2 equiv), dry THF, 60 °C, 2 h; (b) resorcinol (1.0 equiv), 70% H2SO4, rt, 2 h, 35–95% for two steps (a and b); (c) 2-chloro-1-ethanol (1.0 equiv), K2CO3 (3.0 equiv), KI (0.1 equiv), DMF, reflux, 2–10 h, 76–100%; (d) stannous chloride dehydrate (4.0 equiv), DMF, rt, 6 h, 99%; (e) N-methyl-2-oxooxazolidine-3-sulfonamide (2.0 equiv), NEt3 (3.0 equiv), MeCN, 80 °C, 8 h, 99%; (f) ZnCl2 (1.5 equiv), HOAc, reflux, 70 min, 51%; (g) chloromethyl methyl ether (2.0 equiv), K2CO3 (2.5 equiv), acetone, rt, overnight, 84%; (h) CH3I (1.2 equiv), K2CO3 (3.0 equiv), DMF, 80 °C, 30 min, 82%; (i) benzaldehyde or 4-fluorobenzaldehyde (1.05 equiv), 60% KOH aqueous solution (2 mL/mM compound 11), EtOH, 2–5 h, rt; (j) conc. HCl/EtOH = 1:25 (v/v), reflux, 30 min, 80–85% for two steps (i and j); (k) 14 (1.3 equiv), DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (2.0 equiv), anhydrous DCM, rt, 3.5–12 h, 51–85%; (l) 2-methoxyethan-1-ol (1.1 equiv), DBU (2.0 equiv); anhydrous DCM, rt, overnight, 90%.
