Figure 1. Sialic Acid Biosynthetic Pathway.
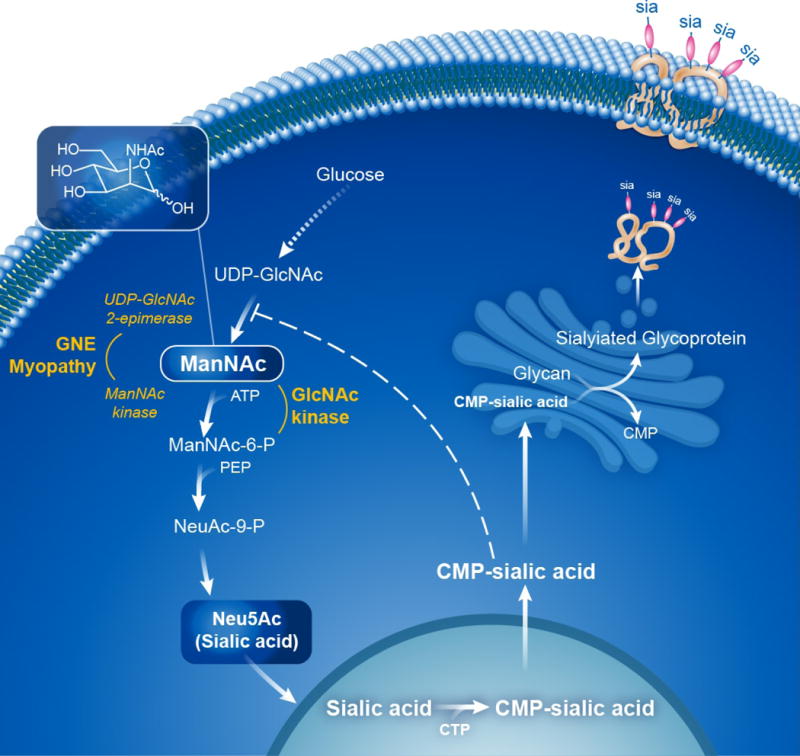
The biosynthesis of Neu5Ac (sialic acid) occurs in the cytosol, where glucose undergoes several modifications to become UDP-GlcNAc. The UDP-GlcNAc 2-epimerase activity of the bifunctional GNE enzyme then epimerises UDP-GlcNAc into ManNAc, after which the GNE ManNAc kinase activity further converts this to ManNAc-6-P, which then undergoes two more steps to form cytoplasmic free sialic acid. A nuclear step activates free sialic acid to CMP-sialic acid, which translocates back to the cytosol, where is utilized by the Golgi complex to sialylate glycans (glycoproteins and glycolipids). Cytoplasmic CMP-sialic acid strongly feedback-inhibits the UDP-GlcNAc 2-epimerase enzymatic activity in its allosteric site (dashed line). PEP=phosphoenolpyruvate. Patients with GNE myopathy have deficiency of GNE UDP-GlcNAc 2-epimerase and ManNAc kinase activity, resulting in decreased sialic acid production. Supplementation of ManNAc (uncharged small molecule that crossed membranes readily) circumvents the feedback inhibition step in this pathway and increases sialic acid production. ManNAc can be converted to ManNAc-6-P by the ancillary enzyme GlcNAc kinase, which likely occurs in GNE myopathy patients with severe ManNAc kinase deficiency. See text for further details and references. Figure courtesy of Daryl Leja and Julia Fekecs, NHGRI, NIH.
