Abstract
Severe and intractable gastrointestinal (GI) bleeding caused by angiodysplasia is a debilitating problem for up to 20% of patients with von Willebrand disease (VWD). Currently, the lack of an optimal treatment for this recurrent problem presents an ongoing challenge for many physicians in their management of affected patients. Over the past few years, studies have pointed to a regulatory role for the hemostatic protein, von Willebrand factor (VWF), in angiogenesis, providing a novel target for the modulation of vessel development. The current article will review the clinical implications and molecular pathology of angiodysplasia in VWD.
Keywords: Angiodysplasia, gastrointestinal bleeding, Heyde’s syndrome, von Willebrand disease, von Willebrand factor
VON WILLEBRAND FACTOR
von Willebrand factor (VWF) is a multimeric plasma glycoprotein that is required for normal hemostasis. It is primarily synthesized in endothelial cells (ECs) from where it is either constitutively secreted or stored as ultra large molecular weight multimers (ULMWM; comprising VWF moieties of 10,000 kD or more) alongside other bioactive molecules in Weibel Palade-bodies (WPBs).1 VWF is also produced in megakaryocytes; however, circulating VWF is almost exclusively derived from ECs.2,3 To render the multimers less thrombogenic, a disintegrin-like and metalloprotease with thrombospondin type 1 motifs 13 (ADAMTS13) cleaves VWF at the A2 domain, thereby reducing VWF multimer size.4
In the circulation, VWF functions as a chaperone of coagulation factor VIII (FVIII) and forms adhesive strings that mediate platelet adhesion and aggregation to sites of vascular damage.5 In addition to playing an integral role in hemostasis, recent research has revealed that VWF functions in several other important biological processes such as angiogenesis, cell proliferation, and inflammation.6,7 Individuals with abnormal or absent VWF are commonly afflicted with excessive bleeding tendencies due to von Willebrand disease (VWD) or acquired von Willebrand syndrome (AVWS), and may be prone to abnormalities in vessel formation, or angiogenesis.
VON WILLEBRAND DISEASE
von Willebrand disease (VWD) was first described in 1926 by Dr. Erik von Willebrand, when he cared for a family with a severe mucocutaneous bleeding problem.8 The index case, a 14 year old female, bled to death during her fourth menstrual period. In the decades since Dr. von Willebrand noted this “pseudohemophilia”, developments in hemostasis and molecular genetics have paved the way for significant progress in our understanding of the disease and allowed for the development of effective treatments. It is now recognized that VWD is an autosomally inherited bleeding disorder, affecting approximately 1% of the population, with a 0.1% symptomatic prevalence.9,10 The disease arises from defective or dysfunctional VWF and, consequently, patients present with excessive mucocutaneous bleeding and, in some severe cases, spontaneous bleeding into joints and soft tissues.11
The International Society on Thrombosis and Hemostasis (ISTH) currently classifies VWD by either quantitative (types 1 and 3) or qualitative (type 2) defects in VWF.3 Type 1 VWD is generally inherited in an autosomal dominant manner and represents 65 – 80% of VWD cases.12 Type 1 VWD is most often caused by a heterozygous missense mutation in the VWF gene that results in a mild to moderate reduction of VWF.13 Type 2 VWD constitutes 20–35% of patients and reflects abnormalities in platelet, collagen or FVIII binding.14 There are four variants of type 2 VWD – 2A (platelet dependent function loss associated with loss of high molecular weight multimers (HMWM) of VWF), 2B (increased binding of VWF to GPIbα), 2M (decreased binding of VWF to GPIbα and/or collagen, but not associated with loss of HMW VWF), and 2N (decreased binding of VWF to FVIII).12 Lastly, type 3 VWD is inherited in an autosomal recessive fashion and is the least common (0.5 – 4 per million in developed countries) and most severe form of the disease.12 Affected individuals are generally homozygous or compound heterozygous for null alleles and have undetectable levels of VWF and substantially reduced levels of FVIII (undetectable to ~5%)13,18; consequently, these patients have a more severe clinical phenotype than the other VWD subtypes.
VWD and Angiodysplasia
Vascular malformations have been documented in VWD patients since the 1960s when Dr. Armand Quick first reported the presence of dilated blood vessels or telangectasias in the noses of several patients.17 We now acknowledge that patients with VWD have an increased frequency of angiodysplasia and vessel abnormalities have also been identified in their gastrointestinal (GI) tract, nail bed, prostate, and skin.18–21 Moreover, when compared with other bleeding disorders, there is a 99% positive predictive value for VWD when there is a high degree of capillary torquation, dilation, and extravasation of blood.19
This association between angiodysplasia and GI bleeding in VWD patients was first reported in 1976 by Ramsey et al., when they described “gastrointestinal vascular dysplasia as a cause of persistently recurring melena in two VWD patients”.22 Though decades have elapsed since this observation, an effective treatment for GI bleeding still evades researchers, and angiodysplasia remains a serious complication for many VWD patients. In fact, recurrent GI bleeding as a result of angiodysplasia is one of the most challenging problems faced by some patients.9,17,23–25
In the GI tract, the presentation of angiodysplasia most commonly occurs in the cecum and ascending colon, but has also been noted throughout the small intestine and stomach.26,27 Patients often present with multiple lesions in the GI tract that are characterized by a fragile vascular network.28 Disruption of this delicate vascular architecture can result in severe intractable bleeding, often leading to anemia and requiring hospitalization for transfusions of packed red blood cells.29 Consequently, many VWD patients experience a decrease in quality of life.29 While GI bleeding is also present in the general population, it is uncommon (prevalence, 0.83%) and the lesions are usually small with a low risk of bleeding.30
The diagnosis of angiodysplasia can be difficult and often requires invasive techniques such as GI endoscopy. Consequently, the exact prevalence of angiodysplasia in VWD is not known. In a retrospective study of VWD patients with occult or angiodysplastic bleeding in the VWD Prophylaxis Network (PN), angiodysplasia was only confirmed as the cause of GI bleeding in one third of patients.31 Although multiple diagnostic methods were used, the cause of bleeding remained unexplained in most patients, highlighting the struggle to determine bleeding etiology.31
The incidence of angiodysplasia varies across VWD subtypes in patients where angiodysplasia is confirmed as the main source of bleeding. In a landmark survey of 4503 VWD patients, Fressinaud and Meyer identified that angiodysplasia was almost exclusively present in patients lacking HMWM of VWF. 9 Specifically, angiodysplasia was reported in 2% of type 2 VWD patients and 4.5% of type 3 VWD patients.9
ACQUIRED VON WILLEBRAND SYNDROME
AVWS is a rare bleeding disorder due to non-inherited structural and/or functional changes in VWF resulting in hemostatic defects. The condition was first described in 1968 in a case study of a patient with lupus erythematosus.32 In the last few decades, researchers have discovered that AVWS is often caused by lymphoproliferative disorders, cardiovascular defects such as aortic stenosis, or myeloproliferative disorders among other risk factors.33 These conditions can influence VWF levels and/or function by decreasing synthesis, inhibiting VWF, increasing clearance due to binding of paraproteins, and pathologically increasing fluid shear stress resulting in increased proteolysis of VWF by ADAMTS13.34–45 In many patients, treating the underlying condition, if possible, often restores the normal VWF function and results in improvement or resolution of AVWS.
Heyde’s Syndrome
Epistaxis and GI bleeding resulting from angiodysplastic lesions have been reported in individuals with AVWS caused by aortic stenosis and left ventricular assist devices (LVAD). Increased bleeding tendencies in these patients point to a direct role for VWF in the development of angiodysplasia.23,46–50
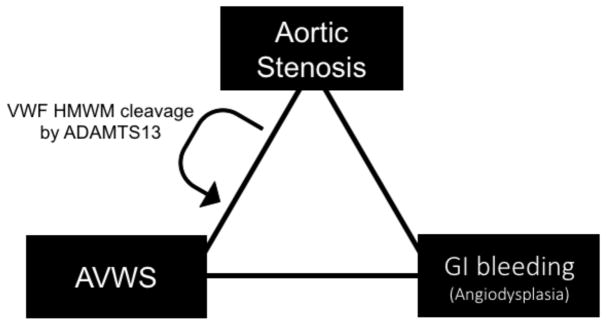
The association between GI bleeding and aortic stenosis was first described in 1958 by E.C. Heyde in a letter to the New England Journal of Medicine. Heyde recounted that at least 10 patients with calcific aortic stenosis had massive gastrointestinal bleeding for which he could discover no cause.51 This triad of conditions, including aortic stenosis, angiodysplasia, and GI bleeding is now eponymously referred to as Heyde’s syndrome (Figure 1).
Figure 1. Heyde’s syndrome refers to the triad of conditions including aortic stenosis, AVWS, and GI bleeding as a result of angiodysplasia.
In individuals with aortic valve stenosis, the aortic valve can be become narrow causing blood jet. This high flow rate often makes VWF more susceptible to cleavage by ADAMTS13 resulting in the loss of HMWM of VWF and AVWS. Consequently, patients can develop angiodysplasia in the GI tract which can lead to severe and intractable bleeding.
In the early 1990s, Warkentin et al., suggested that the link between aortic stenosis and GI bleeding was a deficiency of the largest VWF multimers.52 In patients with aortic stenosis, elevated shear stress modulates VWF from a globular protein to an elongated one in which the A2 domain is unfolded.53 This unfolding exposes cryptic exosites to which ADAMTS13 binds and the VWF multimers are more rapidly cleaved.53 Consequently, patients exhibit a loss of HMWM and develop AVWS. In vitro studies showed that this is often accompanied by abnormalities in platelet adhesion and aggregation.54 Having an aortic valve replacement normalizes the deficiency of VWF HMWM and results in an improvement in AVWS.49,55 King et al., reported the cessation of GI bleeding in 93% of patients with aortic stenosis following an aortic-valve replacement.56
TREATING ANGIODYSPLASTIC GI BLEEDING
The diagnosis and treatment of recurring GI bleeding in VWD is challenging and associated with significant morbidity.9,23,31 Patients frequently experience intractable, bleeding episodes resulting in recurrent hospital admissions and exposure to multiple therapies of limited efficacy. Bleeding episodes can also develop and increase in severity with age.47
Management of GI bleeding can be done via endoscopy or pharmacological methods, but the difficulty in accessing and identifying the angiodysplastic lesions hinders the development and deliverance of effective treatments.31 While acute management of GI bleeding has been successful with VWF replacement therapy, prophylaxis has been less effective for preventing recurrent GI bleeding.23,57 These clinical observations were supported by findings from the VWD PN study, in which prophylaxis was more efficacious at reducing joint bleeding and menorrhagia than GI bleeding.58 This may be because the recovery of normal VWF levels is insufficient to reduce GI bleeding and normalization of VWF in cellular compartments such as ECs, platelets, and subendothelial matrix is also required.59 Furthermore, current VWF replacement treatments are plasma-derived, and may be less effective in preventing recurrent bleeding episodes due to their relative lack of HMWM.26 This loss of HMWM results from proteolytic degradation by ADAMTS-13 during the manufacturing process.60 Given the known association between the development of angiodysplasia and a lack of VWF HMWM, the use of a recombinant VWF replacement product with HMWM and/or even ULMWM could be valuable for the prevention or treatment of recurrent bleeding episodes.60,61
Since VWF replacement therapy is not adequate for preventing recurrent GI bleeding, physicians have attempted alternate treatments. In cases where the angiodysplastic lesions can be identified through endoscopy, embolization of the main vessel in the area and surgical resection can be used to manage bleeding.25,62 This is limited however in cases with multiple, diffuse lesions throughout the GI tract.18 Thalidomide is an inhibitor of neo-angiogenesis, an effect mediated by suppression of vascular endothelial growth factor (VEGF). The use of this agent has been somewhat successful in treating angiodysplasia with or without VWD, but it has been associated with a high incidence of side effects.63–65 Atorvastatin is another drug with anti-angiogenic properties. When administered at high doses (up to 80mg daily), it can also be successful in reducing GI bleeding.63
The clinical difficulties in treating patients with GI bleeding and the continued need for an ideal treatment emphasizes the importance of further research on the regulation of angiogenesis. Elucidating the association between angiodysplasia and abnormalities in VWF on a cellular and molecular level may point to novel therapeutic targets.
CELLULAR AND MOLECULAR BASIS OF ANGIOGENESIS
Angiogenesis is the formation of new, functional blood vessels from the existing vasculature. This is an important process in many physiological events such as embryogenesis, wound-healing, and the menstrual cycle. Deviations of this highly regulated process can give rise to a number of pathological conditions such as proliferative retinopathies, rheumatoid arthritis, and tumourigenesis.66 The dysregulation of angiogenesis is central to the development of angiodysplasia.67
Normally, expansion of the nascent vascular bed arises under hypoxic conditions that require additional vessel growth to satisfy the oxygen requirements of the surrounding tissue. Hypoxia induces signaling through hypoxia-inducible transcription factors (HIFs) that upregulate many angiogenic factors.68 Chiefly, HIF-1α induces VEGF.69 VEGF-A is the best characterized member of the VEGF family of molecules. The binding of VEGF-A to its receptor vascular endothelial growth factor receptor-2 (VEGFR-2) initiates an intracellular signaling cascade prompting EC proliferation and migration toward the hypoxic environment.70
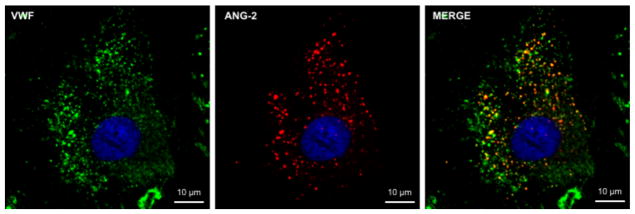
Stabilization and maturation of the newly formed vessel requires a fine balance of the interactions between pro-angiogenic and anti-angiogenic molecules.71 Structural integrity is controlled primarily by Angiopoiein-1 (Ang-1) and Angiopoietin-2 (Ang-2) through signaling of the Tie-2 receptor on ECs.72 Ang-1 is produced in perivascular cells and promotes blood vessel maturity by recruiting pericytes and decreasing vascular leakage.73,74 Ang-2 is synthesized by ECs and stored in WPBs alongside VWF (Figure 2) and is an antagonistic ligand of Tie-2.75 Ang-2 competitively inhibits the binding of Ang-1 at the Tie-2 receptor to promote vessel destabilization and growth by acting synergistically with VEGF.76
Figure 2. Ang-2 is synthesized by ECs and stored in WPBs alongside VWF.
Confocal images of BOECs from healthy control non-VWD individuals with VWF (green), Ang-2 (red), and DAPI nuclear (blue) staining. Ang-2 co-localizes with VWF in yellow WPBs around the nucleus.
Interactions between the extracellular environment (ECM) and ECs are vital for preserving the stability of the newly formed sprout. These interactions are facilitated by cell surface receptors of the integrin family. One member of this family that plays a central role in angiogenesis is integrin αvβ3. This heterodimeric, transmembrane protein is expressed on the surface of ECs and functions as both a pro- and anti-angiogenic molecule through VEGFR-2 signaling depending on the extracellular environment and ligand bound.76,77 Integrin αvβ3 is the best-characterized endothelial receptor for VWF, and is significantly upregulated during wound healing as it can facilitate ligand binding to VEGFR-2.78,79 Further research is still required on this protein as its role appears quite complex and variable depending on the ligand bound.80
Over the years, many molecules have been implicated in the process of angiogenesis, and the list continues to grow with the addition of many chemokines, hormones, neuropeptides, and cytokines among others.81 At every phase of angiogenesis, these molecules work in harmony to prevent pathologic vessel growth.81,82 Angiodysplasia can result from an aberration in this delicate balance between pro- and anti-angiogenic molecules and the association of VWF with angiogenic mediators like Ang-2 has pointed to a regulatory mechanism for this hemostatic protein in angiogenesis.
VWF is a negative regulator of angiogenesis
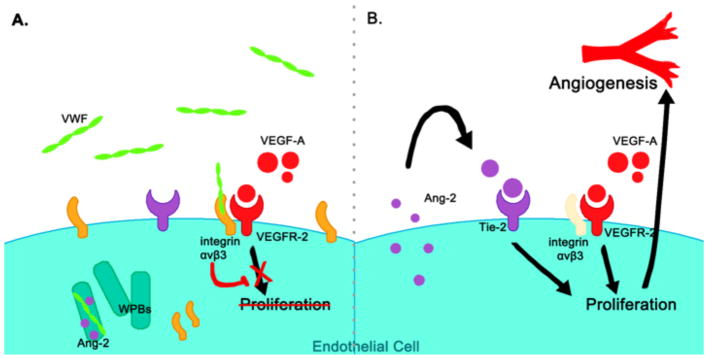
For many years, we have known that VWF defects can play a role in the formation of angiodysplastic lesions. However, the cellular and molecular pathway through which VWF regulates angiogenesis remained relatively unknown until 2011, when Starke and colleagues published an innovative paper pointing to a novel role for VWF as a negative regulator of angiogenesis.28 In this paper, inhibition of VWF expression in human umbilical vein endothelial cells (HUVECs) using short interfering ribonucleic acid (siRNA) caused an increase in VEGF-dependent cell proliferation, migration, and tubule formation.28 The mechanism of VWF regulation was suggested to be through two pathways with intracellular and extracellular components converging to modulate VEGF signaling (Figure 3).
Figure 3. VWF is a negative regulator of angiogenesis28.
(A) VEGF-mediated angiogenesis is regulated by extracellular VWF binding integrin αvβ3 to inhibit VEGFR-2 signaling and by intracellular VWF promoting the formation of WPBs to sequester the pro-angiogenic molecule Ang-2. (B) A lack of VWF allows Ang-2 to escapes the cell to promote EC proliferation, motility, and sprouting via signaling of the Tie-2 receptor.
In the extracellular pathway, VWF influences angiogenesis through interactions with integrin αvβ3. VWF is proposed to inhibit VEGF-2 induced proliferation of endothelial cells when bound to integrin αvβ3. In HUVECs lacking VWF, an internalization of β3 from the surface of cells has been observed. The addition of extracellular VWF only partially rescued the phenotype, suggesting the presence of an intracellular pathway involving VWF. In this intracellular pathway, defective WPB formation in the absence of VWF results in improper storage of Ang-2. Thus, Ang-2 is free to escape the ECs and act on Tie-2 receptors to promote vessel destabilization. In fact, increased Ang-2 release was detected from HUVECs lacking VWF and other studies have found that Ang-2 levels are elevated in the plasma of type 3 VWD patients.28,83 These two pathways are interconnected with previous studies showing that Ang-2 also contributes to the degradation and internalization of αvβ3.84 Furthermore, both pathways are known to influence VEGF signaling,85,86 which is enhanced when they are disturbed in the absence of VWF.28 Starke et al., also showed increased vascularization in the ears of VWF-deficient mice, further validating the role of VWF in angiogenesis.28
In its many interactions with an ever-growing list of molecules, VWF has come to be known as much more than a hemostatic protein.6 The discovery by Starke and colleagues that VWF is a negative regulator of angiogenesis is an important contribution to the existing body of knowledge, as it provides an explanation for the vascular malformations typical of angiodysplasia found in patients with absent or abnormal VWF.
CELLULAR MODELS OF VWD
The anatomic location of endothelial cells within the vascular bed presents a considerable drawback for the routine examination of angiodysplasia and studies of VWD. As such, physicians often use clinical proxies such as malformations in the nail bed capillaries to identify vascular pathologies.19 From a research perspective, this limitation in accessing patients’ ECs has also hindered our understanding of the molecular pathology of VWD, and studies have mainly been done using non-EC based heterologous cell systems.87–94
Human embryonic kidney (HEK) cells can be transfected with recombinant VWF and were preferred models over other cell types (such as COS cells) since they could form pseudo-WPBs capable of exocytosis.89 However, these cells are not native VWF-producing cells, and important factors such as post-translational modifications are potentially altered.92 Furthermore, co-transfections of different mutations may not be a precise reproduction of heterozygous states in vivo.
For many years, HUVECs have also been used to extensively study VWF mutations.95–99 However, it is quite difficult to generate many HUVECs with specific VWF mutations and so the search for a more readily accessible source of ECs continued until 2000 when Dr. Robert Hebbel’s group identified endothelial cells that could be isolated from peripheral blood. These cells, known as blood outgrowth endothelial cells (BOECs), are derived from endothelial progenitor cells (EPCs)100 and have significantly advanced the study of many physiological mechanisms including angiogenesis. BOECs grow with a cobblestone morphology typical of mature endothelial cells and express endothelial specific proteins in culture. Importantly, they are representative of the donor’s native vascular endothelium and maintain their differentiated phenotype through multiple passages.28,94,101,102 Of relevance to VWD studies, BOECs contain and store VWF in WPBs allowing for the examination of cellular properties of VWF such as storage and exocytosis which cannot be achieved using standard VWF functional assays. BOECs are also ideal for studies of angiogenesis, since they can be cultured to form tubules in a three-dimensional matrix and can be grown under shear stress.
VWD STUDIES IN BOECS
To date, four major studies on the properties of BOECs from VWD patients have been published by Dr. Jeroen Eikenboom’s group in the Netherlands and Dr. Anna Randi’s group in England.28,101–103 These studies provide great insight into the role of VWF in the pathology of VWD and angiogenesis.
In 2013, findings on the cellular and molecular basis of VWD using patient-derived BOECs, were published in the same issue of Blood.101,102 Two independent studies showed that it is feasible to isolate and culture BOECs from many patients with VWD, and that BOECs truly are endothelial cells. BOECs isolated from healthy, non-VWD individuals stored VWF normally in elongated WPBs, while BOECs from type 1 and 2 VWD patients had mostly short and rounded WPBs.101 These cellular defects in VWF storage in VWD BOECs also extended to molecular defects in VWF synthesis and processing.102 The heterogenity of VWD was captured in these studies as BOECs derived from patients with similar plama VWF profiles but different mutations had unique cellular VWF phenotypes.102
In addition to uncovering that VWF was a negative regulator of angiogenesis, Starke and colleagues also recapitulated these findings in BOECs isolated from patients with type 1, 2A, and 2M VWD.28 BOECs from VWD patients had reduced VWF levels in culture as well as an associated increase in the release of the pro-angiogenic molecule Ang-2.28 Furthermore, VWD patient-derived cells also showed a significant increase in tube formation on Matrigel®, and increased VEGF-dependent proliferation and migration.28 All of these cellular properties are characteristic of increased angiogenesis and mirror the angiogenic profile of siVWF-treated HUVECs. Interestingly, no differences in angiogenesis were found between the different VWD subtypes. However, this may have been because of the small sample size.
Groeneveld et al., expanded on the aforementioned findings by culturing BOECs from type 1, 2A, 2B, and 3 VWD BOECs and determining their angiogenic characteristics.103 This study highlighted the complexity in distinguishing the influence of different VWF mutations on angiogenesis, given that there was great variation across both control and VWD BOECs. A key observation was the loss of migration directionality in almost all VWD BOECs. While overall, VWD BOECs were not significantly more angiogenic than BOECs from controls, individual patient BOECs showed aberrantly angiogenic phenotypes when compared to controls.
Our research group is the first to successfully culture BOECs from patients of all VWD types and subtypes. Importantly, we have isolated BOECs from multiple type 3 VWD patients (Selvam et al., submitted publication under review). In line with the above-mentioned studies, we have also determined the baseline angiogenic profiles of healthy non-VWD controls, and investigated the role of quantitative and qualitative VWD abnormalities on in vitro surrogates for the development of angiodysplasia. We found that overall there was great variability in the angiogenic properties of both control and VWD BOECs. Common features of BOECs from VWD patients included increased Ang-2 secretion, abnormal cell proliferation, and migration. In particular, type 1 and 3 VWD BOECs had reduced mean cell front migration velocity and increased directionality compared with control BOECs. We found that even within a single VWD type, BOECs from different patients presented with diverse angiogenic characteristics. This finding is consistent with results found by Dr. Eikenboom and Dr. Randi’s groups and highlights the main difficulty in isolating the effects of VWF on angiogenesis in BOECs: inter-individual variability affects all expression profiles including those of many other angiogenic regulators.
Our lab is also interested in understanding the mechanism by which VWF HMWM modulate vessel formation. As previously mentioned, most currently available plasma-derived VWF concentrates lack VWF HMWM, while new recombinant VWF contains the full complement of multimers. BOECs present a unique opportunity to elucidate the mechanisms through which these two different VWF replacement therapy influence angiodysplasia. To accomplish this, our current studies involve treating VWD patient-derived BOECs with plasma-derived VWF and recombinant VWF that has been digested to carry varying levels of multimers and subsequently, performing angiogenesis assays on these treated cells. These experiments have the potential to uncover the role of VWF HMWM in angiogenesis and identify differences in VWF replacement treatment efficacies.
CONCLUSIONS
While much research remains to be done to improve currently available treatments for patients suffering from GI bleeding, our understanding has come a long way since Dr. Erik von Willebrand first brought attention to this complex disease. VWF wears many hats in the vasculature, and its role in angiogenesis provides a potential avenue for treatment for a long fought clinical complication. These initial studies on VWD patient BOECs present a solid foundation from which future studies can be catapulted and holds promise for delineating the pathological mechanisms for different VWF mutations.
Acknowledgments
The authors would like to thank Matt Gordon for his help in obtaining confocal images of BOECs and Lara Casey for designing Figure 3.
Footnotes
CONFLICTS OF INTEREST AND SOURCES OF FUNDING
SNS: None Declared.
PDJ: Receives research funding from: Bayer, CSL Behring, and Octapharma, Honoraria from: Baxalta, Biogen, Octapharma & CSL Behring, and is on advisory boards for: CSL Behring, Baxalta & Biogen.
Contributor Information
Ms. Soundarya Nivedita Selvam, PhD Candidate, Department of Pathology and Molecular Medicine, Queen’s University, Kingston, Canada.
Dr. Paula Denise James, Professor, Department of Pathology and Molecular Medicine and Department of Medicine, Queen’s University, Kingston, Canada.
References
- 1.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J Thromb Haemost. 2012;10(8):1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 4.Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. [PubMed] [Google Scholar]
- 5.Sadler JE. von Willebrand factor assembly and secretion. J Thromb Haemost. 2009;7(Suppl 1):24–27. doi: 10.1111/j.1538-7836.2009.03375.x. [DOI] [PubMed] [Google Scholar]
- 6.Lenting P, Casari C, Christophe O, Denis C. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12):2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 7.Casari C, Lenting PJ, Christophe OD, Denis CV. Von Willebrand Factor Abnormalities Studied in the Mouse Model: What We Learned about VWF Functions. Mediterr J Hematol Infect Dis. 2013;5(1):e2013047. doi: 10.4084/MJHID.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Willebrand EA. Hereditar pseudohemofili. Fin Lakarsallskapets Handl. 1926;67(7):7–112. [Google Scholar]
- 9.Fressinaud E, Meyer D. International survey of patients with von Willebrand disease and angiodysplasia. Thromb Haemost. 1993;70(3):546. [PubMed] [Google Scholar]
- 10.Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010;8(1):213–216. doi: 10.1111/j.1538-7836.2009.03661.x. [DOI] [PubMed] [Google Scholar]
- 11.James PD, Goodeve AC. von Willebrand disease. Genet Med. 2011;13(5):365–376. doi: 10.1097/GIM.0b013e3182035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PD, Lillicrap D. The molecular characterization of von Willebrand disease: good in parts. Br J Haematol. 2013;161(2):166–176. doi: 10.1111/bjh.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: Results from a Canadian cohort study. Blood. 2007;109(1):145–154. doi: 10.1182/blood-2006-05-021105.. [DOI] [PubMed] [Google Scholar]
- 14.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 15.Bowman M, Tuttle A, Notley C, et al. The genetics of Canadian type 3 von Willebrand disease: further evidence for co-dominant inheritance of mutant alleles. J Thromb Haemost. 2013;11(3):512–520. doi: 10.1111/jth.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodeve A, James P. von Willebrand Disease. Genet Med. 2011;13(5):365–76. doi: 10.1097/GIM.0b013e3182035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quick AJ. Telangiectasia: its relationship to the Minot-von Willebrand syndrome. Am J Med Sci. 1967;254(5):585–601. doi: 10.1097/00000441-196711000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Franchini M, Mannucci PM. Von Willebrand disease-associated angiodysplasia: a few answers, still many questions. Br J Haematol. 2013;161(2):177–182. doi: 10.1111/bjh.12272. [DOI] [PubMed] [Google Scholar]
- 19.Koscielny JK, Latza R, Mürsdorf S, et al. Capillary microscopic and rheological dimensions for the diagnosis of von Willebrand disease in comparison to other haemorrhagic diatheses. Thromb Haemost. 2000;84(6):981–988. [PubMed] [Google Scholar]
- 20.Lemesh RA. Case report: recurrent hematuria and hematospermia due to prostatic telangiectasia in classic von Willebrand’s disease. Am J Med Sci. 1993;306(1):35–36. doi: 10.1097/00000441-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Conlon CL, Weinger RS, Cimo PL, Moake JL, Olson JD. Telangiectasia and von Willebrand’s disease in two families. Ann Intern Med. 1978;89(6):921–924. doi: 10.7326/0003-4819-89-6-921. [DOI] [PubMed] [Google Scholar]
- 22.Ramsay DM, Buist TAS, Macleod DAD, Heading RC. Persistent Gastrointestinal Bleeding Due to Angiodysplasia of the gut in von Willebrand Disease. Lancet. 1976;308(7980):275–278. doi: 10.1016/s0140-6736(76)90729-7. [DOI] [PubMed] [Google Scholar]
- 23.Castaman G, Federici AB, Tosetto A, et al. Different bleeding risk in type 2A and 2M von Willebrand disease: a 2-year prospective study in 107 patients. J Thromb Haemost. 2012;10(4):632–638. doi: 10.1111/j.1538-7836.2012.04661.x. [DOI] [PubMed] [Google Scholar]
- 24.Warkentin TE, Moore JC, Anand SS, Lonn EM, Morgan DG. Gastrointestinal bleeding, angiodysplasia, cardiovascular disease, and acquired von Willebrand syndrome. Transfus Med Rev. 2003;17(4):272–286. doi: 10.1016/s0887-7963(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 25.Makris M. Gastrointestinal bleeding in von Willebrand disease. Thromb Res. 2006;118(Suppl):S13–7. doi: 10.1016/j.thromres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Franchini M, Mannucci PM. Gastrointestinal angiodysplasia and bleeding in von Willebrand disease. Thromb Haemost. 2014;112(3):427–431. doi: 10.1160/TH13-11-0952. [DOI] [PubMed] [Google Scholar]
- 27.Boley SJ, Sammartano R, Adams A, DiBiase A, Kleinhaus S, Sprayregen S. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72(4 Pt 1):650–660. [PubMed] [Google Scholar]
- 28.Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117(3):1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanon E, Vianello F, Casonato A, Girolami A. Early transfusion of factor VIII/von Willebrand factor concentrates seems to be effective in the treatment of gastrointestinal bleeding in patients with von Willebrand type III disease. Haemophilia. 2001;7(5):500–503. doi: 10.1046/j.1365-2516.2001.00543.x. [DOI] [PubMed] [Google Scholar]
- 30.Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90(4):564–567. [PubMed] [Google Scholar]
- 31.Makris M, Federici AB, Mannucci PM, et al. The natural history of occult or angiodysplastic gastrointestinal bleeding in von Willebrand disease. Haemophilia. 2014;21(3):338–42. doi: 10.1111/hae.12571. [DOI] [PubMed] [Google Scholar]
- 32.Simone JV, Cornet JA, Abildgaard CF. Acquired von Willebrand’s Syndrome in Systemic Lupus Erythematosus. Blood. 1968;31(6):806–12. [PubMed] [Google Scholar]
- 33.Federici AB, Rand JH, Bucciarelli P, et al. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84(2):345–349. [PubMed] [Google Scholar]
- 34.Fricke WA, Brinkhous KM, Garris JB, Roberts HR. Comparison of inhibitory and binding characteristics of an antibody causing acquired von Willebrand syndrome: an assay for von Willebrand factor binding by antibody. Blood. 1985;66(3):562–569. [PubMed] [Google Scholar]
- 35.Lazarchick J, Pappas AA, Kizer J, Hall SA. Acquired von Willebrand syndrome due to an inhibitor specific for von Willebrand factor antigens. Am J Hematol. 1986;21(3):305–314. doi: 10.1002/ajh.2830210310. [DOI] [PubMed] [Google Scholar]
- 36.Mohri H, Ohkubo T. Acquired von Willebrand’s syndrome due to an inhibitor of IgG specific for von Willebrand’s factor in polycythemia rubra vera. Acta Haematol. 1987;78(4):258–264. doi: 10.1159/000205889. [DOI] [PubMed] [Google Scholar]
- 37.Goudemand J, Samor B, Caron C, Jude B, Gosset D, Mazurier C. Acquired type II von Willebrand’s disease: demonstration of a complexed inhibitor of the von Willebrand factor-platelet interaction and response to treatment. Br J Haematol. 1988;68(2):227–233. doi: 10.1111/j.1365-2141.1988.tb06194.x. [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM, Lombardi R, Bader R, et al. Studies of the pathophysiology of acquired von Willebrand’s disease in seven patients with lymphoproliferative disorders or benign monoclonal gammopathies. Blood. 1984;64(3):614–621. [PubMed] [Google Scholar]
- 39.van Genderen PJ, Vink T, Michiels JJ, van ’t Veer MB, Sixma JJ, van Vliet HH. Acquired von Willebrand disease caused by an autoantibody selectively inhibiting the binding of von Willebrand factor to collagen. Blood. 1994;84(10):3378–3384. [PubMed] [Google Scholar]
- 40.Coleman R, Favaloro EJ, Soltani S, Keng TB. Acquired von Willebrand disease: potential contribution of the VWF:CB to the identification of functionally inhibiting auto-antibodies to von Willebrand factor. J Thromb Haemost. 2006;4(9):2085–2088. doi: 10.1111/j.1538-7836.2006.02072.x. [DOI] [PubMed] [Google Scholar]
- 41.Guerin V, Ryman A, Velez F. Acquired von Willebrand disease: potential contribution of the von Willebrand factor collagen-binding to the identification of functionally inhibiting auto-antibodies to von Willebrand factor: a rebuttal. J Thromb Haemost. 2008;6(6):1051–1052. doi: 10.1111/j.1538-7836.2008.02967.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsai H, Sussman I, Nagel R. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83(8):2171–9. [PubMed] [Google Scholar]
- 43.Wu T, Lin J, Cruz MA, Dong J, Zhu C. Force-induced cleavage of single VWFA1A2A3 tridomains by ADAMTS-13. Blood. 2010;115(2):370–8. doi: 10.1182/blood-2009-03-210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida K, Tobe S, Kawata M, Yamaguchi M. Acquired and Reversible von Willebrand Disease With High Shear Stress Aortic Valve Stenosis. Ann Thorac Surg. 2006;81(2):490–494. doi: 10.1016/j.athoracsur.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 45.Michiels JJ, Schroyens W, Berneman Z, van der Planken M. Acquired von Willebrand syndrome type 1 in hypothyroidism: reversal after treatment with thyroxine. Clin Appl Thromb Hemost. 2001;7(2):113–115. doi: 10.1177/107602960100700206. [DOI] [PubMed] [Google Scholar]
- 46.Schödel J, Obergfell A, Maass AH. Severe aortic valve stenosis and nosebleed. Int J Cardiol. 2007;120(2):286–287. doi: 10.1016/j.ijcard.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 47.Veyradier A, Balian A, Wolf M, et al. Abnormal von Willebrand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120(2):346–353. doi: 10.1053/gast.2001.21204. [DOI] [PubMed] [Google Scholar]
- 48.Pareti FI, Lattuada A, Bressi C, et al. Proteolysis of von Willebrand Factor and Shear Stress–Induced Platelet Aggregation in Patients With Aortic Valve Stenosis. Circulation. 2000;102(11):1290–1295. doi: 10.1161/01.cir.102.11.1290. [DOI] [PubMed] [Google Scholar]
- 49.Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349(4):343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 50.Slaughter MS. Hematologic Effects of Continuous Flow Left Ventricular Assist Devices. J Cardiovasc Transl Res. 2010;3(6):618–624. doi: 10.1007/s12265-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 51.Heyde E. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196. [Google Scholar]
- 52.Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand’s disease the link? Lancet. 1992;340(8810):35–37. doi: 10.1016/0140-6736(92)92434-h. [DOI] [PubMed] [Google Scholar]
- 53.Crawley JTB, de Groot R, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118(12):3212–21. doi: 10.1182/blood-2011-02-306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panzer S, Badr Eslam R, Schneller A, et al. Loss of high-molecular-weight von Willebrand factor multimers mainly affects platelet aggregation in patients with aortic stenosis. Thromb Haemost. 2009;103(2):408–414. doi: 10.1160/TH09-06-0391. [DOI] [PubMed] [Google Scholar]
- 55.Massyn MW, Khan SA. Heyde syndrome: a common diagnosis in older patients with severe aortic stenosis. Age Ageing. 2009;38(3):267–70. doi: 10.1093/ageing/afp019. [DOI] [PubMed] [Google Scholar]
- 56.King RM, Pluth JR, Giuliani ER. The association of unexplained gastrointestinal bleeding with calcific aortic stenosis. Ann Thorac Surg. 1987;44(5):514–516. doi: 10.1016/s0003-4975(10)62112-1. [DOI] [PubMed] [Google Scholar]
- 57.Berntorp E, Windyga J, Group EWS. Treatment and prevention of acute bleedings in von Willebrand disease--efficacy and safety of Wilate, a new generation von Willebrand factor/factor VIII concentrate. Haemophilia. 2009;15(1):122–130. doi: 10.1111/j.1365-2516.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 58.Abshire TC, Federici AB, Alvárez MT, et al. Prophylaxis in severe forms of von Willebrand’s disease: results from the von Willebrand Disease Prophylaxis Network (VWD PN) Haemophilia. 2013;19(1):76–81. doi: 10.1111/j.1365-2516.2012.02916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mannucci PM, Pareti FI, Holmberg L, Nilsson IM, Ruggeri ZM. Studies on the prolonged bleeding time in von Willebrand’s disease. J Lab Clin Med. 1976;88(4):662–671. [PubMed] [Google Scholar]
- 60.Plaimauer B, Schlokat U, Turecek PL, et al. Recombinant von Willebrand Factor: Preclinical Development. Semin Thromb Hemost. 2001;27(4):395–404. doi: 10.1055/s-2001-16892. [DOI] [PubMed] [Google Scholar]
- 61.Mannucci PM, Kempton C, Millar C, et al. Pharmacokinetics and safety of a novel recombinant human von Willebrand factor manufactured with a plasma-free method: a prospective clinical trial. Blood. 2013;122(5):648–657. doi: 10.1182/blood-2013-01-479527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris ES, Hampton KK, Nesbitt IM, Preston FE, Thomas EG, Makris M. The management of von Willebrand’s disease-associated gastrointestinal angiodysplasia. Blood Coagul Fibrinolysis. 2001;12(2):143–148. doi: 10.1097/00001721-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Nomikou E, Tsevrenis V, Gafou A, Bellia M, Theodossiades G. Type IIb von Willebrand disease with angiodysplasias and refractory gastrointestinal bleeding successfully treated with thalidomide. Haemophilia. 2009;15(6):1340–1342. doi: 10.1111/j.1365-2516.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- 64.Bauditz J, Schachschal G, Wedel S, Lochs H. Thalidomide for treatment of severe intestinal bleeding. Gut. 2004;53(4):609–612. doi: 10.1136/gut.2003.029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alikhan R, Keeling D. Von Willebrand disease, angiodysplasia and atorvastatin. Br J Haematol. 2010;149(1):159–160. doi: 10.1111/j.1365-2141.2009.08031.x. [DOI] [PubMed] [Google Scholar]
- 66.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280(6):C1358–66. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 67.Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada J-R. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94(4):1070–1076. doi: 10.1111/j.1572-0241.1999.01017.x. [DOI] [PubMed] [Google Scholar]
- 68.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 69.Semenza GL, Banai S, Shweiki D, et al. Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med. 1996;6(5):151–157. doi: 10.1016/1050-1738(96)00039-4. [DOI] [PubMed] [Google Scholar]
- 70.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 71.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 72.Hansen TM, Singh H, Tahir TA, Brindle NP. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal. 2010;22(3):527–532. doi: 10.1016/j.cellsig.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senger D, Galli S, Dvorak A, Perruzzi C, Harvey V, Dvorak H. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 74.Thurston G, Suri C, Smith K, et al. Leakage-Resistant Blood Vessels in Mice Transgenically Overexpressing Angiopoietin-1. Science. 1999;286(5449):2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 75.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 76.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 77.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J, Roth R, Heuser JE, Sadler JE. Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress. Blood. 2009;113(7):1589–1597. doi: 10.1182/blood-2008-05-158584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96(4):1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Curr Opin Cell Biol. 2008;20(5):514–519. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 82.Potente M, Gerhardt H, Carmeliet P, et al. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 83.Randi AM, Laffan MA, Starke RD. Von Willebrand factor, angiodysplasia and angiogenesis. Mediterr J Hematol Infect Dis. 2013;5(1):e2013060. doi: 10.4084/MJHID.2013.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas M, Felcht M, Kruse K, et al. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J Biol Chem. 2010;285(31):23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99(17):11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 87.Wang JW, Eikenboom J. Von Willebrand disease and Weibel-Palade bodies. Hamostaseologie. 2010;30(3):150–155. [PubMed] [Google Scholar]
- 88.Wang JW, Valentijn KM, de Boer HC, et al. Intracellular Storage and Regulated Secretion of Von Willebrand Factor in Quantitative Von Willebrand Disease. J Biol Chem. 2011;286(27):24180–24188. doi: 10.1074/jbc.M110.215194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michaux G, Hewlett LJ, Messenger SL, et al. Analysis of intracellular storage and regulated secretion of 3 von Willebrand disease–causing variants of von Willebrand factor. Blood. 2003;102(7):2452–2458. doi: 10.1182/blood-2003-02-0599. [DOI] [PubMed] [Google Scholar]
- 90.Haberichter SL, Allmann AM, Jozwiak MA, Montgomery RR, Gill JC. Genetic alteration of the D2 domain abolishes von Willebrand factor multimerization and trafficking into storage. J Thromb Haemost. 2009;7(4):641–650. doi: 10.1111/j.1538-7836.2009.03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeanneau C, Avner P, Sultan Y. Use of monoclonal antibody and colloidal gold in E.M. localization of von Willebrand factor in megakaryocytes and platelets. Cell Biol Int Rep. 1984;8(10):841–848. doi: 10.1016/0309-1651(84)90067-5. [DOI] [PubMed] [Google Scholar]
- 92.Haberichter SL, Budde U, Obser T, Schneppenheim S, Wermes C, Schneppenheim R. The mutation N528S in the von Willebrand factor (VWF) propeptide causes defective multimerization and storage of VWF. Blood. 2010;115(22):4580–4587. doi: 10.1182/blood-2009-09-244327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castaman G, Giacomelli S, Jacobi P, Schneppenheim R. Homozygous type 2N R854W von Willebrand factor is poorly secreted and causes a severe von Willebrand disease phenotype. J Thromb Haemost. 2010;8(9):2011–2016. doi: 10.1111/j.1538-7836.2010.03971.x. [DOI] [PubMed] [Google Scholar]
- 94.Berber E, James P, Hough C, Lillicrap D. An assessment of the pathogenic significance of the R924Q von Willebrand factor substitution. J Thromb Haemost. 2009;7(10):1672–1679. doi: 10.1111/j.1538-7836.2009.03551.x. [DOI] [PubMed] [Google Scholar]
- 95.Booyse F, Quarfoot A, Chediak J, Stemerman M, Maciag T. Characterization and properties of cultured human von Willebrand umbilical vein endothelial cells. Blood. 1981;58(4):788–796. [PubMed] [Google Scholar]
- 96.Ewenstein BM, Inbal A, Pober JS, Handin RI. Molecular Studies of von Willebrand Disease: Reduced von Willebrand Factor Biosynthesis, Storage, and Release in Endothelial Cells Derived From Patients With Type I von Willebrand Disease. Blood. 1990;75(7):1466–1472. [PubMed] [Google Scholar]
- 97.Federici AB, de Groot PG, Moia M, Ijsseldijk MJ, Sixma JJ, Mannucci PM. Type I von Willebrand disease, subtype “platelet low”: decreased platelet adhesion can be explained by low synthesis of von Willebrand factor in endothelial cells. Br J Haematol. 1993;83(1):88–93. doi: 10.1111/j.1365-2141.1993.tb04636.x. [DOI] [PubMed] [Google Scholar]
- 98.de Groot PG, Federici AB, de Boer HC, d’Alessio P, Mannucci PM, Sixma JJ. von Willebrand factor synthesized by endothelial cells from a patient with type IIB von Willebrand disease supports platelet adhesion normally but has an increased affinity for platelets. Proc Natl Acad Sci U S A. 1989;86(10):3793–3797. doi: 10.1073/pnas.86.10.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levenet RB, Booyse FM, Chediak J, Zimmerman ’ TS, Livingstont DM, Lyncht DC. Expression of abnormal von Willebrand factor by endothelial cells from a patient with type IIA von Willebrand disease*. Med Sci. 1987;84:6550–6554. doi: 10.1073/pnas.84.18.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang JW, Bouwens EA, Pintao MC, et al. Analysis of the storage and secretion of von Willebrand factor in blood outgrowth endothelial cells derived from patients with von Willebrand disease. Blood. 2013;121(14):2762–2772. doi: 10.1182/blood-2012-06-434373. [DOI] [PubMed] [Google Scholar]
- 102.Starke RD, Paschalaki KE, Dyer CEF, et al. Cellular and molecular basis of von Willebrand disease: studies on blood outgrowth endothelial cells. Blood. 2013;121(14):2773–2784. doi: 10.1182/blood-2012-06-435727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groeneveld DJ, van Bekkum T, Dirven RJ, et al. Angiogenic characteristics of blood outgrowth endothelial cells from patients with von Willebrand disease. J Thromb Haemost. 2015;13(10):1854–1866. doi: 10.1111/jth.13112. [DOI] [PubMed] [Google Scholar]