Abstract
Background
Symptoms of urinary incontinence are commonly perceived to vary over time; yet, there is limited quantitative evidence regarding the natural history of urinary incontinence, especially over the long-term.
Objective
To delineate the course of urinary incontinence symptoms over time, using two large cohorts of middle-age and older women, with data collected over 10 years.
Study Design
We studied 9,376 women from the Nurses’ Health Study, age 56–81 years at baseline, and 7,491 women from Nurses’ Health Study II, age 39–56 years, with incident urinary incontinence in 2002–3. Urinary incontinence severity was measured by the Sandvik severity index. We tracked persistence, progression, remission, and improvement of symptoms over 10 years. We also examined risk factors for urinary incontinence progression using logistic regression models.
Results
Among women age 39–56 years, 39% had slight, 45% had moderate, and 17% had severe urinary incontinence at onset. Among women age 56–81 years, 34% had slight, 45% had moderate, and 21% had severe urinary incontinence at onset. Across ages, most women reported persistence or progression of symptoms over follow-up; few (3–11%) reported remission. However, younger women and women with less severe urinary incontinence at onset were more likely to report remission or improvement of symptoms.
We found that increasing age was associated with higher odds of progression only among older women (age 75–81 versus 56–60 years, odds ratio=1.84, 95% confidence interval: 1.51, 2.25). Among all women, higher body mass index was strongly associated with progression (younger women: odds ratio=2.37, 95% confidence interval: 2.00, 2.81 body mass index ≤30 vs. < 25 kg/m2; older women: odds ratio=1.93, 95% confidence interval: 1.62, 2.22). Additionally, greater physical activity was associated with lower odds of progression to severe urinary incontinence (younger women: odds ratio=0.86, 95% confidence interval: 0.71, 1.03, highest vs. lowest quartile of activity; older women: odds ratio=0.68, 95% confidence interval: 0.59, 0.80).
Conclusions
Most women with incident urinary incontinence continued to experience symptoms over 10 years; few had complete remission. Identification of risk factors for urinary incontinence progression, such as body mass index and physical activity, could be important for reducing symptoms over time.
Keywords: urinary incontinence, natural history, Sandvik severity index
Introduction
Urinary incontinence (UI) is a common health condition in women which adversely impacts perceived health1 and is strongly associated with depression and social isolation.2–5 A review of several epidemiologic studies estimated up to 40% prevalence of UI in older women living in the community.6–8 While UI is commonly perceived as a dynamic condition with symptoms that vary over time, there is limited quantitative evidence regarding the natural history of UI in patients, especially over the long-term.9 There are large prospective cohorts that have examined urinary symptoms in women over many years (i.e., at least 6 to 10 years10–17) and the age range of the women studied has been limited, which may restrict generalizability of these findings. Therefore, it is difficult for clinicians to discuss with patients the likely course of their incontinence, and for clinicians and patients to appropriately consider treatment options. Thus, to provide data on the natural history of UI, we utilized data from the Nurses’ Health Study I & II, two large prospective cohorts of middle-aged and older women, with repeated measures of UI.
Our objective was to delineate the natural course of symptoms in women with incident UI followed over 10 years. More specifically, our goal was to better understand whether symptoms of new UI persist, progress, improve or remit, and whether age at onset, initial UI severity, and other risk factors influence the course of symptoms, and finally, risk factors for UI progression.
Materials and Methods
Study Population
The Nurses’ Health Study (NHS) was initiated in 1976 when 121,700 female registered nurses ages 30–55 years responded to a mailed questionnaire about their medical history and lifestyle. Using similar methodology, the Nurses’ Health Study II (NHS II) was initiated in 1989. The target population included women between 25–42 years old; the upper age was selected to correspond with the lowest age in NHS. A total of 116,430 women were enrolled in NHS II. Both cohorts utilize identical methods for data collection and follow-up, including biennial mailed questionnaires to update health and lifestyle information. During each questionnaire cycle, full-length questionnaires are sent in initial mailings, followed by abbreviated questionnaires to maximize participation. To date, the follow-up rate in both cohorts is approximately 90%. The Institutional Review Board of Brigham and Women’s Hospital approved both the Nurses’ Health Study and Nurses’ Health Study II.
Urinary Incontinence
To obtain information on UI, women were asked, “During the past 12 months, how often have you leaked or lost control of your urine?” Response choices were never, < 1/month, 1/month, 2–3/month, approximately 1/week, and almost every day. Women reporting any UI were then asked, “When you lose your urine, how much usually leaks?” Response options were a few drops, enough to wet your underwear, enough to wet your outer clothing, and enough to wet the floor. A reliability study among a subgroup of these nurses demonstrated high reproducibility of responses to these questions.18
UI severity was measured by the Sandvik severity index, using the two questions described above. The Sandvik index is well-validated.19 We calculated severity by multiplying the reported frequency of UI by the amount of leakage.19 Frequency of UI was assigned a value from 1 to 4, with both <1/month and 1/month assigned a score of 1, and subsequently higher numbers indicating greater frequency. The amount of leakage was assigned a value of 1 for drops or 2 for more than drops. After multiplying scores for frequency and amount, women with a total score of 1 to 2 were classified as having slight UI severity, those with a score of 3 to 4 were classified as having moderate UI severity, and those with a score of 6 or more with severe UI.
For these analyses, we utilized similar context and available follow-up for both cohorts; we identified new onset of UI beginning in 2002 for the NHS cohort and 2003 for the NHS II cohort (i.e., after excluding women who reported having UI in the preceding time period, 2000 (NHS) and 2001 (NHS II)). To track UI symptoms over time, we utilized data on persistence, progression, remission, and improvement of UI symptoms over 10 years after this first report of symptoms, using information on UI from participants in NHS in 2004, 2008, 2012, and in NHS II in 2005, 2009, 2013. “Persistence” was defined as reporting the same severity of UI at onset and at 2 or more subsequent follow-up points (i.e., the majority of follow-up points). “Progression” was defined as reporting a worse severity of UI at 2 or more follow-up points after onset. “Remission” was defined as reporting no UI at 2 or more follow-up points, and “improvement” was defined as either reporting no UI or less severe UI at 2 or more follow-up points than at onset. A small group of participants had patterns which did not conform to any of these definitions, and were classified as having “inconsistent patterns” of UI. Since we had many years of follow-up, and multiple repeated measures of UI, there was some overall variability in the patterns; thus, to help broadly interpret patterns and summarize the data collected, we chose to define these natural history categories based on participants’ reports at the majority of follow-up points.
Measurement of Risk Factors
To examine risk factors for UI progression, we used information from the cohort questionnaires in 2002 (NHS) and 2003 (NHS II) on a wide variety of demographic, health, and lifestyle factors, including age, race, height and weight, reproductive history, smoking, physical activity, exogenous hormone use, history of vascular conditions, and hysterectomy. Information on physical activity was collected from NHS participants in 2000 and from NHS II participants in 2001, thus we used these reports; women reported the number of hours spent on various leisure activities (e.g., walking, running) during the past year, and total energy expenditure was calculated in metabolic-equivalent task hours per week, which has been previously described in detail.20 In addition, women reported a history of diagnoses of stroke, myocardial infarction, and type 2 diabetes.
Population for Analysis
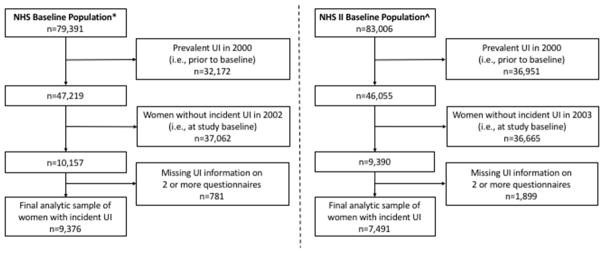
Of the original 121,700 Nurses’ Health Study participants, 79,391 were alive and responded to UI questions on the 2002 long questionnaire. Since our focus here is new UI in 2002, we excluded women who did not have UI symptoms in 2002 and further excluded women who had UI in 2000. We included the 9,376 women who provided UI information on at least 2 additional questionnaires during the period from 2004 through 2012. For 1,232 of these women only missing UI data on one questionnaire during follow-up, we carried forward their reports from the previous questionnaire (after testing that the large majority of women with complete data had similar reports on consecutive questionnaires). (Figure 1)
Figure 1.
Flow chart of eligible participants for the analytic sample of women with incident UI.
*Baseline population consisted of NHS participants who were alive and responded to the UI questions on the 2002 questionnaire. ^Baseline population consisted of NHS II participants who were alive and responded to the UI questions on the 2003 questionnaire.
NHS: Nurses’ Health Study
NHS II: Nurses’ Health Study II
UI: urinary incontinence
Of the original 116,430 Nurses’ Health Study II participants, 83,006 were alive at baseline for these UI analyses and responded to UI questions on the 2003 long questionnaire. We excluded women who did not have UI symptoms in 2003 and further excluded women who had UI in 2001. We included the 7,491 women who provided UI information on at least 2 additional questionnaires during the period from 2005 through 2013. For 1,750 women only missing UI data on one questionnaire during follow-up, we carried forward their reports from the previous questionnaire. (Figure 1) To test the impact of carrying forward reports for women with missing values, we also conducted analyses only including women with no missing data; since findings were extremely similar, we present all results carrying forward data if relevant.
Statistical Analysis
Descriptive statistics (mean, standard deviation, or percentage) were used to evaluate women’s baseline demographic and health characteristics, after standardizing to the age distribution in each cohort.
To evaluate risk factors related to progression of UI, we used multivariable-adjusted logistic regression models to estimate odds ratios (OR) of progression from slight or moderate UI to severe UI symptoms, separately in each cohort. We considered the following variables, based on the literature regarding risk factors for UI incidence: age, race, body mass index (BMI), parity, smoking, physical activity, postmenopausal hormone use, history of vascular disease, history of type 2 diabetes, and prior hysterectomy. The status of these factors was determined based on the participants’ report in 2002 for NHS and 2003 for NHS II, when UI symptoms were first reported. We performed analyses both with and without adjusting for baseline Sandvik Severity Index in the multivariable-adjusted logistic regression models, since UI severity could be related to both these risk factors and to likelihood of UI progression; effect estimates for risk factors did not meaningfully differ between models and thus we report results without adjusting for baseline Sandvik Severity Index. We calculated 95% confidence interval (CIs) for all estimates, and evaluated linear tests of trend for ordinal variables. In addition, in secondary analyses, to evaluate risk factors that predict remission of UI symptoms, we used identical methods as those described above to estimate ORs of remission to no UI, separately in each cohort among all women with incident UI at baseline.
Results
Among women with incident UI, age 39–56 years (from NHS II), 39% had slight, 45% had moderate, and 17% had severe UI at onset. Among women with incident UI, age 56–81 years (from NHS), 34% had slight, 45% had moderate, and 21% had severe UI. Table 1 presents age-adjusted characteristics of these participants at UI onset. Among the younger women, those with severe UI compared to those with slight or moderate UI had worse health profiles; they had higher BMI, were somewhat more likely to be current smokers, had higher prevalence of type 2 diabetes, and were more likely to have had a prior hysterectomy. Among the older women, those with severe UI at onset also had higher BMI and were somewhat more likely to be current smokers, as well as lower physical activity levels and higher prevalence of type 2 diabetes; they also had greater prevalence of prior hysterectomy compared to older women with slight or moderate UI.
Table 1.
Age-adjusted baseline characteristics for women with urinary incontinence in the Nurses’ Health Studies
| Women 39–56 years of agea | Women 56–81 years of ageb | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Slight UI (n=2891) | Moderate UI (n=3348) | Severe UI (n=1252) | Slight UI (n=3206) | Moderate UI (n=4196) | Severe UI (n=1974) | |
| Mean Age, yrsc | 48.9±4.6 | 49.0±4.5 | 49.3±4.4 | 66.4±6.5 | 66.5±6.5 | 67.2±6.4 |
| Race, % | ||||||
| White | 98 | 96 | 97 | 98 | 98 | 98 |
| Black | 1 | 2 | 1 | 1 | 1 | 1 |
| Asian | 1 | 2 | 2 | 1 | 1 | 1 |
| Mean BMI, kg/m2 | 26.7±6.0 | 28.0±6.5 | 29.6±7.3 | 26.5±4.9 | 27.4±5.4 | 28.6±5.8 |
| Parity, % | ||||||
| 0 | 18 | 16 | 14 | 6 | 5 | 5 |
| 1–2 | 55 | 54 | 55 | 36 | 36 | 35 |
| 3+ | 27 | 30 | 31 | 58 | 60 | 60 |
| Smoking, % | ||||||
| Never | 65 | 65 | 62 | 47 | 46 | 45 |
| Past | 27 | 27 | 28 | 47 | 47 | 47 |
| Current | 8 | 8 | 11 | 6 | 8 | 8 |
| Mean Physical Activity, METs/week | 20.5±26.2 | 18.4±25.0 | 18.2±23.0 | 19.6±22.8 | 17.3±20.3 | 15.6±20.0 |
| Hysterectomy, % | 20 | 22 | 25 | 41 | 43 | 47 |
| Diabetes, % | 3 | 5 | 6 | 8 | 9 | 13 |
| History of vascular disease, %d | 2 | 2 | 3 | 5 | 5 | 7 |
| Postmenopausal hormone use, % | ||||||
| Never | 12 | 14 | 13 | 20 | 20 | 17 |
| Past | 13 | 13 | 13 | 36 | 36 | 41 |
| Current | 17 | 16 | 18 | 44 | 43 | 41 |
| Premenopausal (not eligible for hormone therapy) | 58 | 57 | 55 | 1 | 1 | 1 |
| Diuretic use, % | 7 | 8 | 8 | 15 | 16 | 16 |
UI, urinary incontinence; yrs, years; BMI, body mass index; kg, kilogram; m, meters; METs, metabolic equivalent of task
Values are means±SD or percentages and are standardized to the age distribution of the study population.
Nurses’ Health Study II participants, baseline characteristics measured in 2003.
Nurses’ Health Study participants, baseline characteristics measured in 2002.
Value is not age-adjusted.
History of vascular disease includes myocardial infarction or stroke.
When we examined UI symptoms over 10 years (Table 2), overall patterns were fairly similar for both younger and older participants. Among younger women with slight UI, 81% reported continued symptoms -- either persistence of slight symptoms or progression to moderate or severe UI at the majority of follow-up points, although symptoms remained slight for the majority (62%) of younger women and very few progressed to severe UI (4%). Among older women, 83% of those with slight UI at onset reported some UI over the 10 year follow-up period; 61% had persistence of slight UI symptoms, while 6% progressed to severe UI.
Table 2.
Natural history of urinary incontinence symptoms over 10 years in incident casesa, according to urinary incontinence symptom severity at onset
| Women 39–56 years of age (NHS II) | Women 56–81 years of age (NHS) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sandvik Severity Index at Onset | Slight UI | Moderate UI | Severe UI | Slight UI | Moderate UI | Severe UI |
|
| ||||||
| UI cases | 2891 | 3348 | 1252 | 3206 | 4196 | 1974 |
|
| ||||||
| N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | |
| Persistence of UI symptoms at same severity as onsetb | 1808 (62) | 967 (29) | 546 (44) | 1945 (61) | 1217 (29) | 950 (48) |
| Progression of UI symptomsb | 538 (19) | 414 (12) | NA | 699 (22) | 702 (17) | NA |
| Progression to Moderate UI | 441 (15) | NA | NA | 516 (16) | NA | NA |
| Progression to Severe UI | 97 (4) | 414 (12) | NA | 183 (6) | 702 (17) | NA |
| Remission and Improvement of UI symptomsb | 323 (11) | 1725 (52) | 706 (56) | 229 (7) | 1889 (45) | 1024 (52) |
| Remission to no UI | 323 (11) | 286 (9) | 54 (4) | 229 (7) | 196 (5) | 64 (3) |
| Improvement to no UI or Slight UIc | NA | 1725 (52) | 319 (26) | NA | 1889 (45) | 411 (21) |
| Improvement to no UI, Slight UI, or Moderate UId | NA | NA | 706 (56) | NA | NA | 1024 (52) |
| Inconsistent patterns of UI symptomsb | 222 (8) | 242 (7) | 0 (0) | 333 (10) | 388 (9) | 0 (0) |
UI, urinary incontinence; NHS, Nurses’ Health Study; NA, not applicable
Incident UI cases in 2003 for NHS II and 2002 for NHS.
Patterns of UI symptoms were defined according to UI symptom categories at majority of follow-up points (i.e., 2+/3 time points) after UI onset
Numbers in this row also include women from the above row
Numbers in this row also include women from the above two row
Among the younger women whose initial UI was moderate, 41% had persistence of moderate UI or progression to severe UI. The majority (52%) of younger women with moderate UI had remission or improvement of their UI symptoms over 10 years; however, it is notable that only 9% reported complete remission – that is, among younger women with new onset of moderate UI symptoms, 91% still had some continuing symptoms during follow-up. Among the older women whose initial UI was moderate, 29% had persistence of moderate UI and 17% reported progression to severe UI. Fewer (45%) of older women with moderate UI had remission or improvement, with only 5% reporting complete remission of UI symptoms during follow-up.
Among younger women whose incident UI was severe, 44% had persistently severe symptoms over follow-up and slightly over half reported remission or improvement of their symptoms; only 4% had complete symptom remission, such that, in total 96% continued to report some level of UI during follow-up. Among older women with incident, severe UI symptoms, 48% continued to have severe UI during follow-up, and slightly over half reported remission or improvement of their symptoms. Only 3% had complete symptom remission, with a total of 97% who continued to report some level of UI during follow-up.
Overall, although UI severity over time was somewhat variable, UI symptoms persisted during the subsequent 10 years in both younger and older women with incident UI, and worse UI at onset was indicative of higher likelihood of continued symptoms.
Risk Factors for UI Progression, or Remission
In addition to considering the natural history of UI symptoms, we examined risk factors for UI progression from mild or moderate to severe symptoms, separately in younger and older women. After multivariable adjustment (Table 3), we found that increasing age was associated with increasing odds of progression among older women, but not in younger women age. For example, among older women, those 75 years or older had 1.84(95% CI:1.51,2.25) times the odds of progression to severe UI compared to women <60 years of age. However, when comparing the oldest to youngest age groups in the younger cohort, the odds ratio was 1.00(95% CI:0.82,1.23). Increasing BMI was strongly associated with progression to severe UI symptoms in both cohorts (younger participants: OR=2.37, 95% CI:2.00,2.81 comparing women with BMI ≤30 kg/m2 vs. < 25 kg/m2; older participants: OR=1.93, 95% CI:1.62,2.22 for the same comparison). Smoking was also related to increased odds of progression to severe UI; for example, among younger women, current smokers had 1.29 times (95% CI:1.03,1.63) the odds of progression to severe UI compared to never smokers, and among older women the odds ratio for progression to severe UI was 1.65 (95% CI:1.32,2.03). Greater physical activity was generally associated with decreased odds of progression to severe UI (OR=0.86, 95% CI:0.71,1.03 comparing highest vs. lowest quartile in younger women; OR=0.68, 95% CI:0.59,0.80 for older women). Finally, among older women, diabetes was associated with an increased risk of progression to severe UI (OR=1.31, 95% CI:1.11,1.55).
Table 3.
Odds ratios of progression from slight or moderate urinary incontinence to severe urinary incontinence symptoms, according to risk factors
| Women 39–56 years of age (n=7,491) | Women 56–81 years of age (n=9,376) | |||
|---|---|---|---|---|
|
| ||||
| Cases | OR (95% CI) | Cases | OR (95% CI) | |
| Age, yrs | ||||
| <45 | 207 | 1.0 (reference) | - | - |
| 45–<50 | 361 | 0.93 (0.77–1.13) | - | - |
| 50+ | 489 | 1.00 (0.82–1.23) | - | - |
| <60 | - | - | 260 | 1.0 (reference) |
| 60–<65 | - | - | 419 | 1.18 (0.99–1.40) |
| 65–<70 | - | - | 476 | 1.55 (1.30–1.84) |
| 70–<75 | - | - | 394 | 1.82 (1.52–2.19) |
| 75+ | - | - | 286 | 1.82 (1.49–2.23) |
| Race | ||||
| White | 1008 | 1.0 (reference) | 1797 | 1.0 (reference) |
| Black | 22 | 1.71 (1.04–2.81) | 21 | 0.93 (0.57–1.54) |
| Asian | 16 | 1.28 (0.74–2.20) | 10 | 0.77 (0.39–1.52) |
| BMI, kg/m2 | ||||
| < 25 | 285 | 1.0 (reference) | 532 | 1.0 (reference) |
| 25–<30 | 301 | 1.55 (1.30–1.85) | 678 | 1.39 (1.22–1.58) |
| 30+ | 457 | 2.37 (2.00–2.81) | 619 | 1.89 (1.64–2.18) |
| Parity | ||||
| 0 | 175 | 1.0 (reference) | 67 | 1.0 (reference) |
| 1–2 | 558 | 1.00 (0.83–1.21) | 607 | 1.48 (1.12–1.95) |
| 3+ | 324 | 1.12 (0.92–1.37) | 1118 | 1.55 (1.18–2.03) |
| Smoking | ||||
| Never | 644 | 1.0 (reference) | 781 | 1.0 (reference) |
| Past | 308 | 1.15 (0.99–1.33) | 902 | 1.17 (1.05–1.30) |
| Current | 104 | 1.29 (1.03–1.63) | 147 | 1.65 (1.34–2.03) |
| Physical Activity, METs/weeka | ||||
| Quartile 1 | 337 | 1.0 (reference) | 561 | 1.0 (reference) |
| Quartile 2 | 253 | 0.80 (0.67–0.96) | 446 | 0.77 (0.67–0.89) |
| Quartile 3 | 230 | 0.76 (0.63–0.91) | 434 | 0.84 (0.73–0.97) |
| Quartile 4 | 237 | 0.86 (0.71–1.03) | 366 | 0.68 (0.59–0.80) |
| Hysterectomy | 265 | 1.19 (0.97–1.47) | 825 | 1.06 (0.95–1.18) |
| Diabetes | 67 | 1.22 (0.91–1.63) | 232 | 1.29 (1.09–1.53) |
| History of vascular disease | 29 | 1.26 (0.83–1.91) | 117 | 1.04 (0.84–1.30) |
| Postmenopausal hormone use | ||||
| Never | 134 | 1.0 (reference) | 347 | 1.0 (reference) |
| Past | 162 | 1.12 (0.89–1.41) | 718 | 1.11 (0.96–1.29) |
| Current | 184 | 1.04 (0.80–1.34) | 664 | 0.94 (0.80–1.09) |
UI, urinary incontinence; OR, odds ratio; CI, confidence interval; yrs, years; BMI, body mass index; kg, kilogram;
m, meters; METs, metabolic equivalent of task
Cohort specific cutoffs
In additional analyses, we also examined the same risk factors in relation to remission of UI symptoms, separately in younger and older women (results not shown in table). We found that BMI and physical activity were significantly related to remission of UI symptoms. For example, among the younger women, those with BMI ≤30 kg/m2 had lower odds of UI remission compared to women with BMI <25 kg/m2 (OR=0.70, 95% CI: 0.56, 0.86). Similarly, higher BMI was association with lower odds of UI remission among older women (OR=0.71, 95% CI: 0.54, 0.92). Greater physical activity was significantly association with increased odds of UI remission among younger women (OR=1.33, 95% CI: 1.06, 1.67 comparing highest vs. lowest quartile) but not older women (OR=0.94, 95% CI: 0.73, 1.22)
Comment
Overall, patterns of UI symptoms over 10 years were similar for younger and older women. The vast majority of women with incident UI continue to experience UI over 10 years of follow-up, while very few (less than 10%) have remission of UI symptoms. Severity of UI at onset was associated with the likelihood of persistent symptoms; among women with incident severe UI, 96–97% reported continued UI symptoms during follow-up. In this study, two important modifiable risk factors associated with odds of UI progression to severe symptoms were identified, physical activity and BMI. Women in the lowest quartile of physical activity and women who were overweight and obese (BMI >30 kg/m2) when they reported UI symptom onset had an increased odds of symptom progression over 10 years of follow-up.
The majority of prior studies regarding the natural history of urinary incontinence have generally considered two follow-up points,26–26 from which it is difficult to construct a comprehensive description of the natural history. Additionally, many of these studies had limited sample sizes22,23 and short follow-up periods.21–26 In this analysis, we were able to describe patterns of persistence, progression, improvement, and remission of UI symptoms for a large sample of women with new onset of UI, over 10 years of follow-up and over 4 data-intervals.
In general, the findings from our study are consistent with prior studies that have investigated patterns of UI over time. A review of 14 longitudinal studies of UI found that symptoms reported at baseline tended to persist throughout follow-up and remission of symptoms was more likely to occur among younger women.9 The majority of these studies only followed participants for 1 to 2 years and studies with longer follow-up (9–16 years) generally only assessed symptoms at baseline and the end of follow-up.
Studies that have specifically investigated patterns of UI according to severity have found that severe UI is more common among older women8 and that women with severe UI are less likely to experience improvement in symptoms compared to women with mild or moderate UI.27
Overall, estimates for rates of remission of UI symptoms have varied widely (3–40%) across studies, although study designs vary also making it difficult to directly compare results.6,26–27 In general, studies that reported higher rates of remission were conducted among younger women or defined remission as only one report of no UI symptoms. In the current analysis, we found that very few women (3–11%, depending on age and severity of incident UI) experience remission of UI symptoms at more than one time point over 10 years. Similar to prior studies, we found somewhat less remission among older women. Additionally, we had a longer follow-up with a larger sample size across a wider range of ages and more measures of UI over time in our study and thus we were likely able to better capture women who truly experience remission of symptoms versus fluctuations of UI symptoms.
Very few studies have investigated risk factors related to progression of UI symptoms and most have only looked at age.9 In the present analysis, we considered a number of potential risk factors for UI progression and found significant associations for BMI and physical activity; for analyses of UI progression, findings were consistent among both younger and older women. With further research confirming these findings, our results indicate that both older and younger women may be able to alter their likelihood of progressing to severe incontinence by addressing BMI and physical activity, which could be important for eventually creating public health messaging for women who want to reduce the negative impact of incontinence. In the clinical setting among women with slight disease who are not yet bothered by their current symptoms, such messages may provide an avenue for reducing risk of worsening whether patients decide to undergo treatment or not.
A major strength of our study is the multiple, repeated measures of UI over time among both younger and older women. Our study also has several limitations. First, UI information was self-reported; however, we have previously demonstrated higher reliability of self-reported incontinence symptoms among these women,18 and other studies have demonstrated high validity of self-reported incontinence compared to clinical assessment.28 Additionally, our assessment of UI symptoms via mailed questionnaires helps to reduce embarrassment that might otherwise cause underreporting of UI among these women. Secondly, our study population is relatively homogenous, consisting of nurses who are predominantly white. While this may limit generalizability, we would expect accurate reporting of symptoms and health status in this population which strengthens the validity of our findings. Moreover, although our participants are health professionals, in previous research on UI in this cohort, we have consistently found that UI prevalence, incidence, and risk factors are similar to those in other populations, and thus findings here are likely generalizable to Caucasian women. Furthermore, there is no specific reason to suspect that UI patterns would differ among nurses versus other women. Thirdly, we only included women with new onset of UI and excluded those who had reported UI symptoms in the time period before “baseline”, in order to focus on a homogenous and clinically relevant group of women with new symptoms. However, we cannot be absolutely certain that all these incident UI cases had never previously experienced UI since we did not track symptoms for many years prior to baseline; nonetheless, since the present analysis demonstrates that very few women (<7%) have remission of UI symptoms, it is highly likely that the vast majority of UI cases included in our analytic cohort here are indeed incident. Lastly, we do not have information on UI treatment. This may suggest that we may be somewhat underestimating spontaneous remission of reduction of UI symptoms. However, in a sub-study among a sample of participants with frequent UI, we inquired about UI treatments, and only 20% reported treatment, similar to other studies. In calculations to assess sensitivity of our findings, we calculated that if 20% of women who experienced remission had used treatment, the proportion of younger women with spontaneous incontinence remission would only change from 9% to 7% and the proportion of older women with spontaneous incontinence remission would change from 5% to 4%. Thus, the small number of treated women should not meaningfully alter our conclusions. Moreover, we found in our sub-study of participants with frequent UI, that 47% of women who received treatment reported little to no improvement in their UI symptoms. Thus, it is not likely that treatment had a significant impact on our results.
Overall, the vast majority of women with new UI symptoms continue to experience UI over 10 years, while very few have complete remission of UI symptoms. Additionally, we found patterns of UI over 10 years were similar for younger and older women. Identification of potential risk factors for prevention of UI progression, such as BMI and physical activity, could be important for reducing symptoms over time.
Implications and Contributions.
Symptoms of urinary incontinence are commonly perceived to vary over time; yet, there is limited quantitative evidence regarding the natural history of urinary incontinence, especially over the long-term. In this study, we sought to delineate the course of urinary incontinence symptoms over time, using two large cohorts of middle-age and older women, with data collected over 10 years. Most women with incident urinary incontinence continued to experience symptoms over 10 years and few women had complete remission of symptoms. Identification of risk factors for urinary incontinence progression, such as body mass index and physical activity, could be important for reducing symptoms over time.
Acknowledgments
Financial Support: This work was supported by a grant from the National Institutes of Diabetes and Digestive and Kidney Diseases (#R01 DK105050). The Nurses’ Health Study is funded by grants from the National Cancer Institute (#UM1 CA186107 and #P01 CA87969).
We thank the participants and staff of the Nurses’ Health Study and the Nurses’ Health Study II for their valuable contributions.
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
Paper presentation information: This work was presented at the American Urogynecologic Society 38th Annual Scientific Meeting in Providence, Rhode Island, October 3–7, 2017.
Role of funding source: The funding sources had no role in study design; in the collection, analysis and interpretation of data; in writing of the report and in the decision to submit the article for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suskind AM, Cawthon PM, Nakagawa S, et al. Urinary incontinence in older women: The role of body composition and muscle strength: from the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2017;65:42–50. doi: 10.1111/jgs.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melville JL, Fan MY, Rau H, Nygaard IE, Katon WJ. Major depression and urinary incontinence in women: temporal associations in an epidemiologic sample. Am J Obstet Gynecol. 2009;201:490e1–7. doi: 10.1016/j.ajog.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 3.Melville JL, Delaney K, Newton K, Katon W. Incontinence severity and major depression in incontinent women. Obstet Gynecol. 2005;106:585–92. doi: 10.1097/01.AOG.0000173985.39533.37. [DOI] [PubMed] [Google Scholar]
- 4.Sung VW, West DS, Hernandez AL, Wheeler TL, 2nd, Myers DL, Subak LL Program to Reduce Incontinence by Diet and Exercise (PRIDE) Association between urinary incontinence and depressive symptoms in overweight and obese women. Am J Obstet Gynecol. 2009;200:557.e1–5. doi: 10.1016/j.ajog.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip SO, Dick MA, McPencow AM, Martin DK, Ciarleglio MM, Erekson EA. The association between urinary and fecal incontinence and social isolation in older women. Am J Obstet Gynecol. 2013;208:146e1–7. doi: 10.1016/j.ajog.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62:16–23. doi: 10.1016/s0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 7.Landefeld CS, Bowers BJ, Feld AD, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449–58. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 8.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S Norwegian EPINCONT study. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–7. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 9.Irwin DE, Milsom I, Chancellor MB, Kopp Z, Guan Z. Dynamic progression of overactive bladder and urinary incontinence symptoms: a systematic review. Eur Urol. 2010;58:532–43. doi: 10.1016/j.eururo.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol. 2007;165:309–18. doi: 10.1093/aje/kwk018. [DOI] [PubMed] [Google Scholar]
- 11.Byles J, Millar CJ, Sibbritt DW, Chiarelli P. Living with urinary incontinence: a longitudinal study of older women. Age ageing. 2009;38:333–8. doi: 10.1093/ageing/afp013. discussion 251. [DOI] [PubMed] [Google Scholar]
- 12.Jahanlu D, Hunskaar S. The Hordaland Women’s Cohort: prevalence, incidence, and remission of urinary incontinence in middle-aged women. Int Urogynecol J. 2010;21:1223–9. doi: 10.1007/s00192-010-1172-7. [DOI] [PubMed] [Google Scholar]
- 13.Erekson EA, Cong X, Townsend MK, Ciarleglio MM. Ten-Year Prevalence and Incidence of Urinary Incontinence in Older Women: A Longitudinal Analysis of the Health and Retirement Study. J Am Geriatr Soc. 2016;64:1274–80. doi: 10.1111/jgs.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428–34. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 15.Komesu YM, Schrader RM, Ketai LH, Roger RG, Dunvian GC. Epidemiology of mixed, stress and urgency incontinence in middle-aged/older women: the importance of incontinence history. Int Urogynecol J. 2016;27:763–62. doi: 10.1007/s00192-015-2888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komesu YM, Schrader RM, Rogers RG, Ketai LH. Urgency urinary incontinence in women 50 years or older: incidence, remission, and predictors of change. Female Pelvic Med Reconstr Surg. 2011;17:17–23. doi: 10.1097/SPV.0b013e31820446e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komesu YM, Rogers RG, Schrader RM, Lewis CM. Incidence and remission of urinary incontinece in a community-based population of women >50 years. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:581–9. doi: 10.1007/s00192-009-0838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thom DH, Brown JS, Schembri M, Ragins AI, Subak LL, Ven Den Eeden SK. Incidence of and risk factors for change in urinary incontinence status in a prospective cohort of middle-aged and older women: the reproductive risk of incontinence study in Kaiser. J Urol. 2010;184:1394–401. doi: 10.1016/j.juro.2010.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497–9. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognition in women with type 2 diabetes. Am J Epidemiol. 2009;170:1040–7. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahanlu D, Hunskaar S. Type and severity of new-onset urinary incontinence in middle-aged women: the Hordaland Women’s Cohort. Neurourol Urodyn. 2011;30:87–92. doi: 10.1002/nau.20966. [DOI] [PubMed] [Google Scholar]
- 22.Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. J Urol. 1991;146:1255–9. doi: 10.1016/s0022-5347(17)38063-1. [DOI] [PubMed] [Google Scholar]
- 23.Samuelsson EC, Victor FT, Svärdsudd KF. Five-year incidence and remission rates of female urinary incontinence in a Swedish population less than 65 years old. Am J Obstet Gynecol. 2000;183:568–74. doi: 10.1067/mob.2000.106763. [DOI] [PubMed] [Google Scholar]
- 24.Wehrberger C, Temml C, Ponholzer A, Madersbacher S. Incidence and remission of female urinary incontinence over 6. 5 years: analysis of a health screening project. Eur Urol. 2006;50:327–32. doi: 10.1016/j.eururo.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Nygaard IE, Lemke JH. Urinary incontinence in rural older women: prevalence, incidence and remission. J Am Geriatr Soc. 1996;44:1049–54. doi: 10.1111/j.1532-5415.1996.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson SL, Scholes D, Boyko EJ, Abraham L, Fihn SD. Predictors of urinary incontinence in a prospective cohort of postmenopausal women. Obstet Gynecol. 2006;108:855–62. doi: 10.1097/01.AOG.0000236446.17153.21. [DOI] [PubMed] [Google Scholar]
- 27.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 28.Bradley CS, Brown JS, Van Den Eeden SK, Schembri M, Ragins A, Thom DH. Urinary incontinence self-report questions: reproducibility and agreement with bladder diary. Int Urogynecol J. 2011;22:1565–71. doi: 10.1007/s00192-011-1503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]