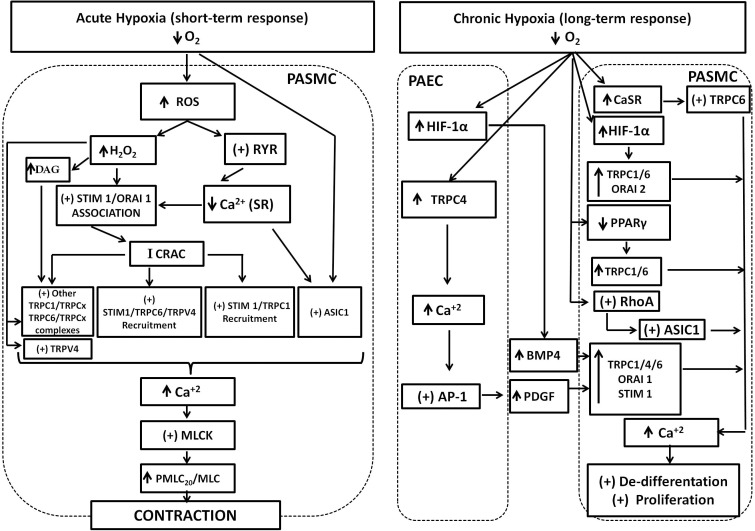
FIGURE 1.
SOC and ROC involvement in the pulmonary response to acute and chronic hypoxia. Acute hypoxia generates increase of ROS and H2O2. ROS stimulates RyR opening and depletion of SR-Ca2+ stores. Both H2O2 and depletion of SR-Ca2+ stores stimulates Stim1/Orai1 association to generate the calcium-release activated calcium current (Icrac), which in turn promotes further interaction of Stim1 with TRPC1, TRPC6, and TRPV4 proteins and recruitment to generate the store operated calcium (Isoc) current. The participation of other TRPC proteins in association with TRPC1 or 6 to generate Isoc cannot be discarded. Increase of DAG cell content promoted by H2O2 activates TRPC6/TRPCx channels. H2O2 can also directly activate TRPC6 and TRPV4 channels. Finally, ASIC1 also mediates hypoxic increase of SOC through an unknown mechanism. Increase of SOC and ROC results in the final increase of Ca2+, stimulation of the myosin light chain kinase (MLCK), phosphorylation of myosin light chain, and contraction. Chronic hypoxia upregulates hypoxia inducible factor (HIF1) resulting in increased expression the secreted ligand bone morphogenetic protein-4 (BMP-4) from pulmonary artery endothelial cells (PAEC). Hypoxia also upregulates TRPC4 and Ca2+ increase, stimulates activator protein1 (AP-1) that in turn upregulates platelet-derived growth factor (PDGF). Secreted PDGF upregulates TRPC1, 4, 6, Orai1, and stim1 in pulmonary artery smooth muscle cells (PASMC). Hypoxia also upregulates the calcium sensing receptor (CaSR) coupled to TRPC6 stimulation and downregulates PPAR-γ in PASMC. Chronic hypoxia also stimulates RhoA protein, which stimulates ASIC1 incorporation to de membrane. The net result is an increase of expression and activity of TRPC1, 4, 6, Orai 1, 2, and ASIC1. The contribution of all these proteins to store and receptor operated calcium entry results in sustained Ca2+ increase to promote PASMC proliferation and remodeling.