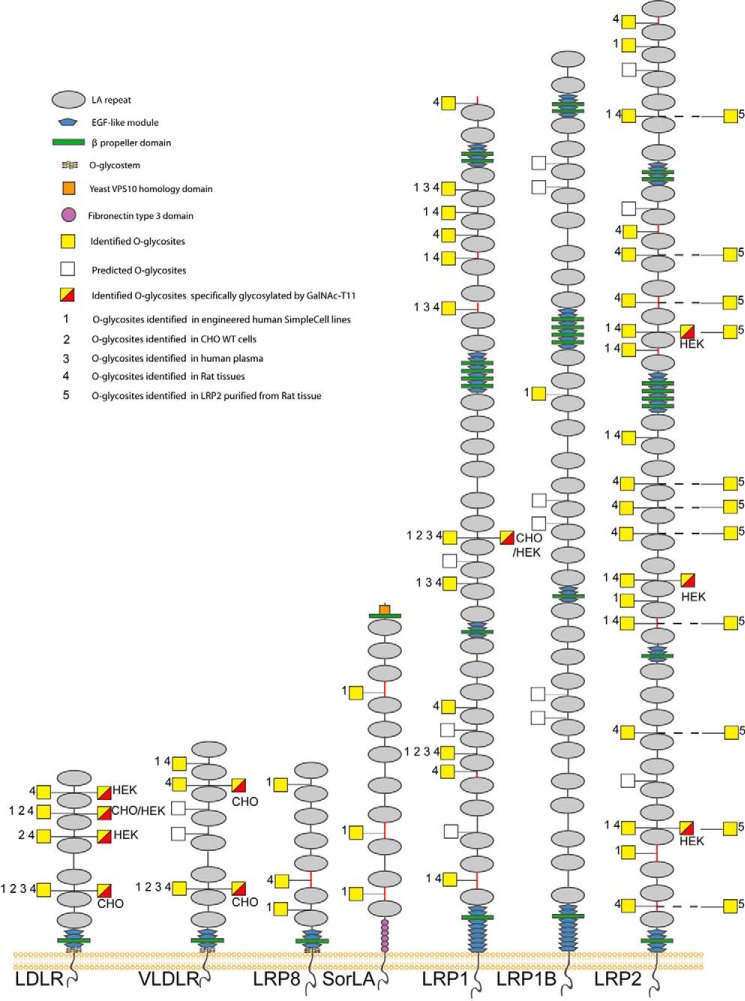
Figure 1.
Graphic depiction of O-glycosites in LA linkers of human LDLR-related proteins. The depiction summarizes all identified O-glycosites (filled yellow squares) and predicted O-glycosites (open squares) in LA linker regions of the LDLR family proteins. LA linkers with the relaxed sequence motif XXC6XnTC1XX (n = 3–5) are indicated as black lines, and other linkers are indicated with red lines. Glycosites previously identified in engineered human SimpleCell lines (labeled 1) (15–17), in CHO WT cells (labeled 2) (18), in human plasma (labeled 3) (19), and in rat liver, kidney, and brain tissues (labeled 4) and purified rat LRP2 (labeled 5) in the present study are shown. Detailed information about O-glycosites is presented in Data Set S1, O-glycopeptides identified in the present study are described in Data Set S2, and (glyco)peptides identified in purified LRP2 are described in Data Set S4. Glycosites specifically regulated by GalNAc-T11 (yellow and red squares) were identified by comparative analysis of isolated shed LDLR expressed in HEK293 SC cells with and without KO of GALNT11 as previously reported (11) or by differential dimethyl-labeling O-glycoproteomics (17) of CHO and HEK293 SC cell lines (indicated) with and without KO of GALNT11 in unpublished studies. Note that some LA modules are not conserved between rat and human (Table S1).