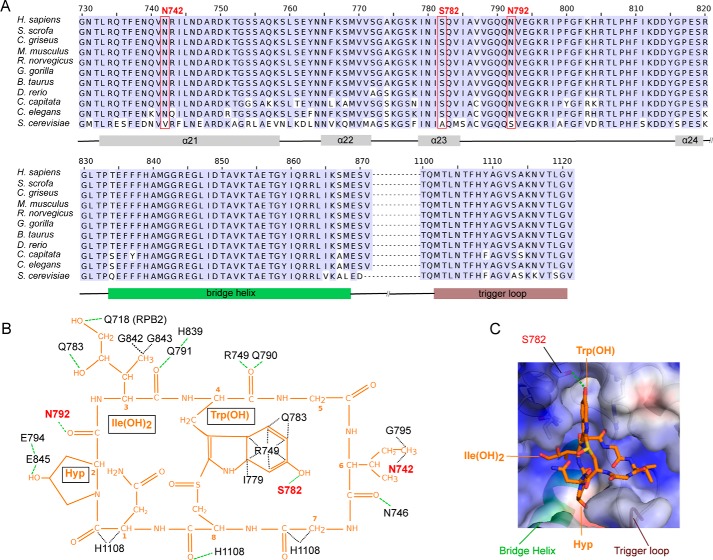
Figure 3.
Interactions of mammalian Pol II with α-amanitin. A, sequence alignment of residues forming the α-amanitin-binding pocket in RPB1 between various metazoan species and the yeast S. cerevisiae (bottom row). The red boxes indicate amino acid residues that form metazoan-specific interactions with α-amanitin. Helices α21 to α24, bridge helix, and trigger loop are indicated at the bottom of the sequence alignment. B, schematic overview of Pol II–amanitin interactions. The chemical structure of α-amanitin is shown in orange. RPB1 residues conserved over eukaryotes are labeled in black, whereas metazoan-specific amanitin-interacting residues are labeled in red. The green dashed lines indicate hydrogen bonds, whereas black dashed lines show other interactions. C, surface representation of the amanitin-binding Pol II pocket. Positively and negatively charged surfaces are in blue and red, respectively. The bridge helix, trigger loop, and RPB1 residue Ser782 are indicated.