Figure 10.
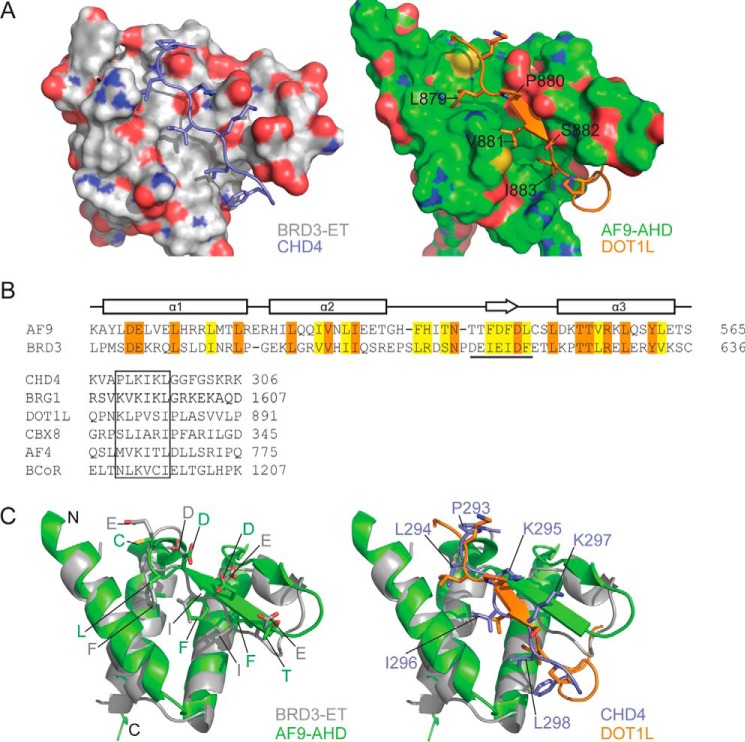
ET interaction mechanism is conserved in AF9/ENL family proteins. A, comparison of BRD3–CHD4 (left) and AF9–DOT1L (right) complexes showing the ET/AHD as a surface and the partner peptide as cartoon/stick representation. B, top, amino acid sequences of the ET and AHD domains of human BRD3 and AF9, respectively. Identical residues are shown in orange and similar residues in yellow. The sequence that forms a β-sheet with the partner is underlined. Bottom, alignment of the ET-binding regions of CHD4 and BRG1 with the corresponding regions from the AHD-binding proteins CBX8, AF4, and BCoR. Sequences from the human proteins are shown in all cases. C, comparison of the interface residues of ET/AHD domains (left) and of the partner peptides (right); strong conservation is apparent in the former case, whereas the alternating pattern of hydrophobic and basic residues observed in ET-binding sequences is not as strongly conserved in AHD-binding sequences.