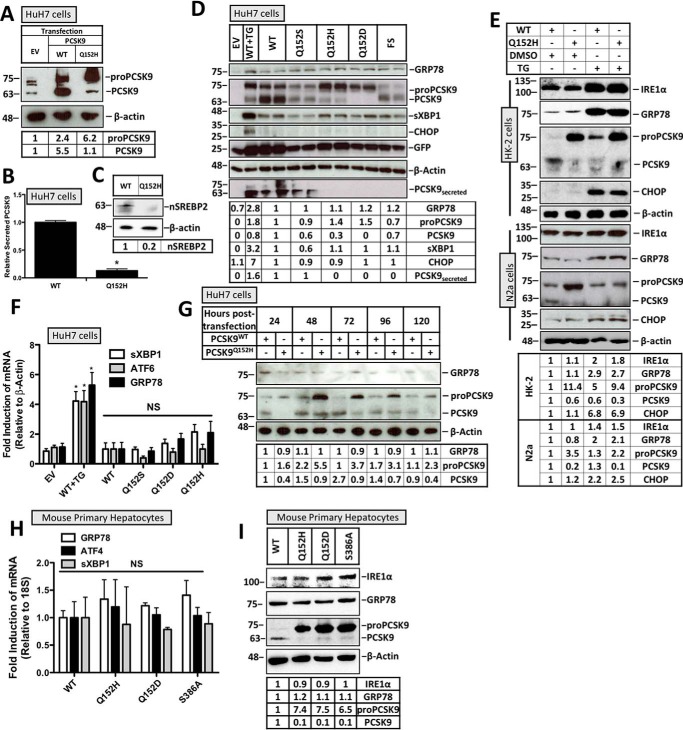
Figure 1.
Retention of PCSK9 variants in the ER does not induce the UPR. HuH7 cells were seeded in DMEM and transfected with either EV control, WT, Q152H, Q152S, Q152H, Q152D, or FS V5-labeled PCSK9 variants for 48 h. Cells were also treated with TG (100 nm) for 24 h to serve as a positive control for UPR activation. In addition to V5-PCSK9 transfection, HuH7 cells were also cotransfected with the ERAI plasmid encoding FLAG-XBP1. A, CMV-driven PCSK9 expression was compared with endogenous PCSK9 expression in HuH7 cells. B, an ELISA was run on the media from HuH7 cells transfected with either PCSK9WT or PCSK9Q152H. C, SREBP2 activation, in the form of nuclear SREBP2 (nSREBP2), was also examined in PCSK9Q152H-transfected cells and compared with controls via immunoblotting. D and F, immunoblot and real-time PCR analyses of these cells were performed for ER stress markers ATF6, GRP78, sXBP1, and CHOP. Immunoblot analysis was also completed on V5-PCSK9 in whole cell lysate and medium to examine PCSK9 maturation and secretion, respectively. GFP, originating from the bicistronic pIRES2-EGFP V5-PCSK9 plasmids, served as a transfection control. E, HK-2 and N2a cells transfected with either PCSK9WT or PCSK9Q152H were also examined for UPR activation in the presence or absence of TG. G, a time course experiment was carried out in HuH7 cells transfected with either PCSK9WT or PCSK9Q152H to identify the time point yielding the greatest level of PCSK9 retention. H and I, mouse primary hepatocytes were also transfected with PCSK9Q152H, PCSK9Q152D, and PCSK9S386A and examined for ER stress marker expression of GRP78, ATF4, sXBP1, and IRE1α via real-time PCR and immunoblotting. Differences between treatments were assessed with unpaired Student's t tests. All values are represented as means with error bars representing S.D. B, *, p < 0.05 versus WT. F, *, p < 0.05 versus WT. F and H, nonsignificant (NS).