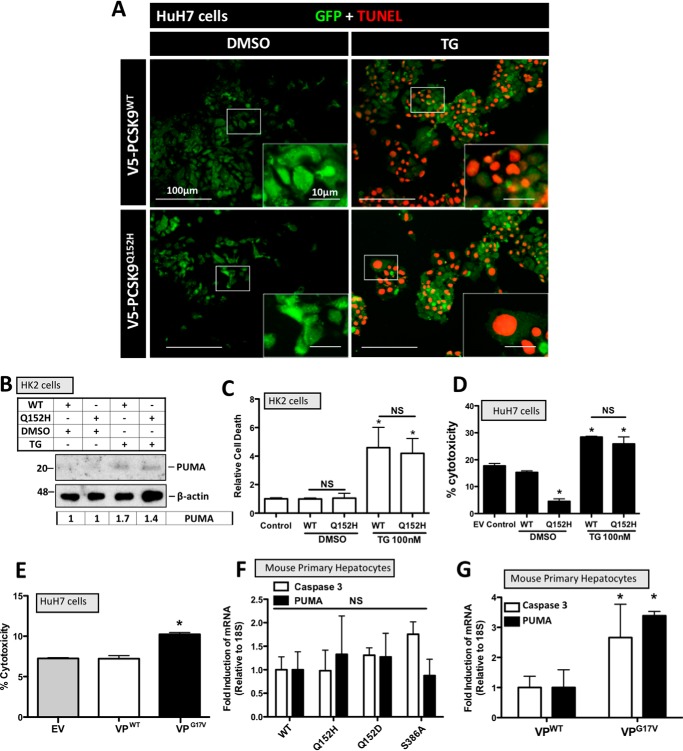
Figure 4.
ER PCSK9 retention does not induce apoptosis. HuH7 and HK-2 cells were seeded in DMEM and transfected with either PCSK9WT or PCSK9Q152H for 48 h. Cells were also treated with TG (100 nm) or DMSO vehicle control for 24 h. A, HuH7 cells were fixed in 4% paraformaldehyde and stained for apoptosis-induced DNA damage using a 594-labeled TUNEL stain (red). Following TUNEL staining, cells were also stained for GFP to identify cells transfected with the bicistronic pIRES2-EGFP V5-PCSK9 plasmids (green). B, transfected HK-2 cells were also examined for the apoptosis marker PUMA via immunoblot analysis. C and D, cell viability assays were carried out on transfected HK-2 and HuH7 cells using trypan blue stain and lactate dehydrogenase assays, respectively. E, a lactate dehydrogenase assay was also carried out on cells transfected with VPG17V, a mutant known to induce ER stress as a result of ER retention (21). F, freshly isolated mouse primary hepatocytes were transfected with PCSK9 variants PCSK9Q152H, PCSK9Q152D, and PCSK9S386A and examined for the apoptosis markers caspase-3 and PUMA via real-time PCR. G, apoptosis marker expression of caspase-3 and PUMA was also examined in VP-transfected mouse primary hepatocyte using real-time PCR. Differences between treatments were assessed with unpaired Student's t tests. All values are represented as means, and error bars represent S.D. C, *, p < 0.05 versus DMSO-treated WT. D, *, p < 0.05 versus DMSO-treated WT. F and G, *, p < 0.05 versus VPWT. C, D, and E, nonsignificant (NS).