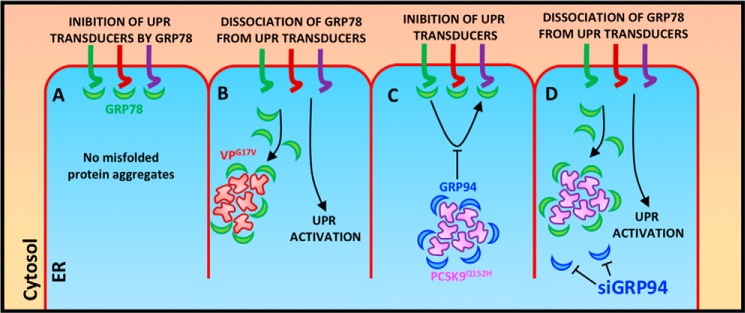
Figure 9.
Model for the masking of ER-resident PCSK9 from UPR sensor GRP78. A, in normal homeostatic conditions, GRP78 is recruited to ER transducers ATF6, IRE1α, and PERK and acts to block UPR activation. B, in the presence of ER-retained protein such as VPG17V, GRP78 dissociates from UPR transducers and interacts with accumulated misfolded proteins to prevent further aggregation. This process liberates ER transducers and leads to UPR activation. C, in contrast to VPG17V, GRP78 is not recruited to ER-retained PCSK9Q152H due to the interaction taking place with GRP94. D, removal of GRP94 from PCSK9 aggregates, however, restores the function of GRP78 as a sensor of UPR activation in response to ER PCSK9 accumulation.