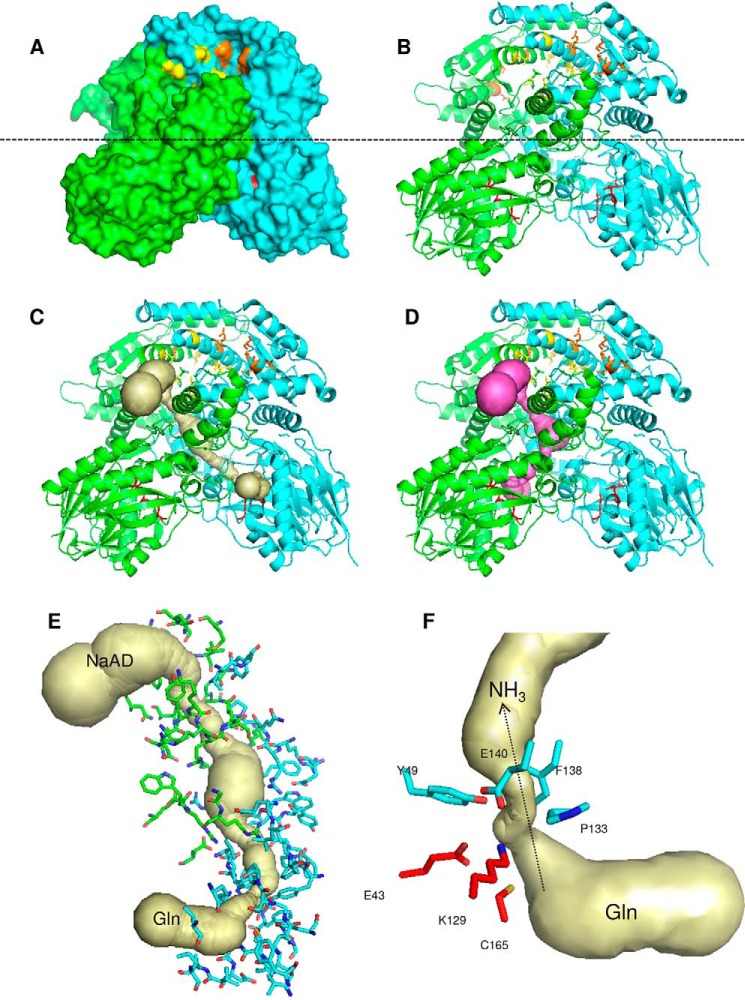
Figure 6.
Structure of the dimeric NadE2Gln from B. thailandensis. A and B, surface (A) and cartoon (B) representations; the monomers are colored green and cyan. The glutaminase catalytic triade residues are in red sticks. Residues involved in NaAD and ATP binding are shown as yellow and orange sticks, respectively. The dashed line indicates the separation of the NAD synthetase domain on the top and the glutaminase domain on the bottom. C and D, the ammonia tunnel connecting the glutaminase and NaAD synthetase domain in different protomers (C) and within the same protomer (D). The detailed structure of the intersubunit ammonia tunnel of the dimeric NadE2Gln from B. thailandensis is shown. E, residues forming the intersubunit ammonia tunnel from different promoters are indicated as green and cyan sticks, respectively. F, conserved residues forming the major constriction within the ammonia tunnel are show in cyan, and the glutaminase catalytic residues are in red. The figures were generated using PyMOL and the PDB entry 4F4H. The ammonia tunnel was identified by CAVER.