Figure 7.
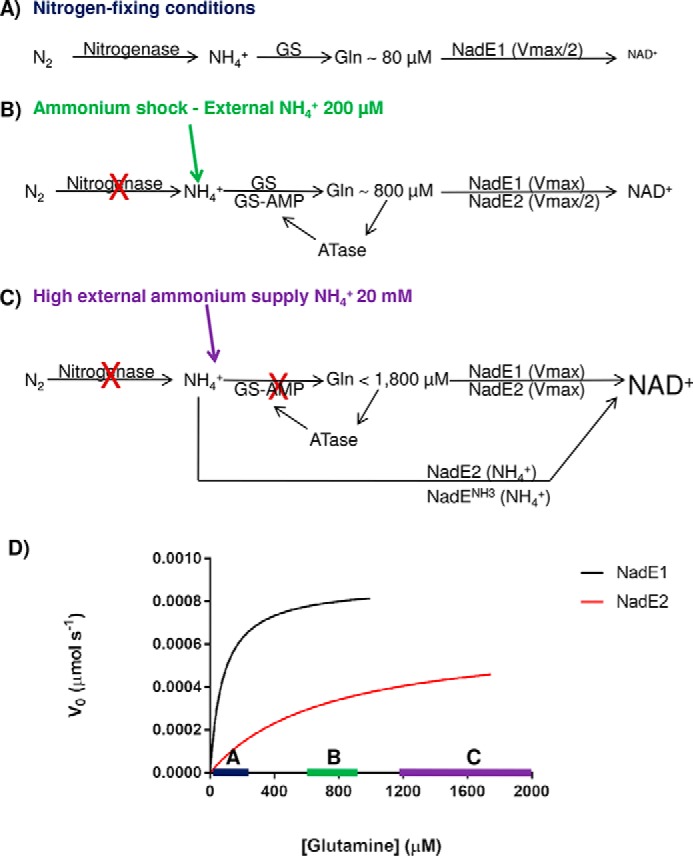
Model for the role of the three NadE in A. brasilense. A, under nitrogen-fixing conditions nitrogenase feeds ammonium into GS, and intracellular levels of glutamine are low (∼80 μm). NadE1Gln operates at Vmax/2 and promptly responds to small fluctuations in glutamine availability within this range (see the blue bar with Michaelis–Menten curves in D). NadE2Gln and NadENH3 activities are negligible leading to slow production of NAD+. B, upon an ammonium shock of NH4+ 200 μm, the intracellular glutamine rises to ∼800 μm, leading to total inhibition of nitrogenase by ADP-ribosylation and nearly full inhibition of GS by adenylylation. NadE1Gln operates at Vmax, whereas NadE2Gln operates at Vmax/2 and promptly responds to small fluctuations in glutamine availability within this range (see the green bar with Michaelis–Menten curves in D). NAD+ production is elevated. C, when external levels of ammonium are high, the high intracellular glutamine will lead to NadE1Gln and NadE2Gln to operate at Vmax using glutamine as substrate. Furthermore, because GS is fully inactive due adenylylation, free intracellular ammonium could be directly assimilated into NAD+ by NadE2Gln and presumably also by NadENH3 bypassing GS activity. The concert action of all three isoforms of NadE is likely to further enhance NAD+ production. D, velocities of the different A. brasilense NadE isoforms accordingly to the intracellular glutamine levels.
