Abstract
Purpose
Practice guidelines recommend that patients who receive neoadjuvant chemotherapy and radiation for locally advanced rectal cancer complete postoperative adjuvant systemic chemotherapy, irrespective of tumor downstaging.
Patients and Methods
The National Comprehensive Cancer Network (NCCN) Colorectal Cancer Database tracks longitudinal care for patients treated at eight specialty cancer centers across the United States and was used to evaluate how frequently patients with rectal cancer who were treated with neoadjuvant chemotherapy also received postoperative systemic chemotherapy. Patient and tumor characteristics were examined in a multivariable logistic regression model.
Results
Between September 2005 and December 2010, 2,073 patients with stage II/III rectal cancer were enrolled in the database. Of these, 1,193 patients receiving neoadjuvant chemoradiotherapy were in the analysis, including 203 patients not receiving any adjuvant chemotherapy. For those seen by a medical oncologist, the most frequent reason chemotherapy was not recommended was comorbid illness (25 of 50, 50%); the most frequent reason chemotherapy was not received even though it was recommended or discussed was patient refusal (54 of 74, 73%). After controlling for NCCN Cancer Center and clinical TNM stage in a multivariable logistic model, factors significantly associated with not receiving adjuvant chemotherapy were age, Eastern Cooperative Oncology Group performance status ≥ 1, on Medicaid or indigent compared with private insurance, complete pathologic response, presence of re-operation/wound infection, and no closure of ileostomy/colostomy.
Conclusion
Even at specialty cancer centers, a sizeable minority of patients with rectal cancer treated with curative-intent neoadjuvant chemoradiotherapy do not complete postoperative chemotherapy. Strategies to facilitate the ability to complete this third and final component of curative intent treatment are necessary.
INTRODUCTION
In 2009, an estimated 40,870 new cases of rectal cancer were diagnosed in the United States.1 Treatment strategies for patients with stage II/III rectal cancer have evolved over the past two decades to include neoadjuvant chemoradiotherapy followed by surgery and postoperative adjuvant chemotherapy. There are limited data to define an optimal adjuvant chemotherapy regimen, although select reports have suggested a trend toward improved disease-free survival and overall survival with fluorouracil-based adjuvant chemotherapy.2–6
Practically, however, this therapeutic approach can be challenging. After 6 weeks of arduous combined modality treatment and then a major operation, there are some patients who are reluctant to proceed to complete the final phase of treatment (ie, systemic adjuvant chemotherapy). Patients and physicians may be reluctant to proceed to complete therapy when tolerance of neoadjuvant treatment was poor, when there were surgical complications, or, alternatively, when surgical pathology has already demonstrated a dramatic response to the neoadjuvant therapy.7–13 For example, approximately 20% of patients have a complete pathologic response to induction neoadjuvant chemoradiotherapy.7,14–17 In this situation, patients and physicians may forego further systemic therapy.
The National Comprehensive Cancer Network (NCCN) Colorectal Cancer Outcomes (CRC) Database project was initiated in 2005 to evaluate the outcomes of cancer care, practice patterns, and adherence to evidence-based guidelines as a continuum in collaboration among eight of the 21 NCCN cancer centers. A recent analysis evaluating concordance with NCCN CRC Guidelines and the American Society of Clinical Oncology quality measures demonstrated relatively low mean concordance rates (81%) for adjuvant chemotherapy in patients with clinical stage II/III rectal cancer within 9 months of diagnosis.18 This article reports an analysis of multiple variables from the NCCN CRC database to determine the significant factors that would predict the omission of adjuvant therapy after standard neoadjuvant chemoradiotherapy and surgery.
PATIENTS AND METHODS
Study Cohort
Patients with locally advanced rectal cancer presenting to the eight participating NCCN institutions between September 1, 2005, and December 31, 2010, were selected. Participating institutions included City of Hope Comprehensive Cancer Center, Dana-Farber Cancer Institute, Fox Chase Cancer Center, Memorial Sloan-Kettering Cancer Center, The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Solove Research Institute, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Roswell Park Cancer Institute, and The University of Texas MD Anderson Cancer Center.
Data Collection
Data were abstracted from the medical records of eligible patients with rectal cancer longitudinally from the time of diagnosis. Eligibility criteria included patients ≥ 18 years old with a clinical diagnosis of locally advanced rectal cancer as per the American Joint Committee on Cancer (seventh edition) who received adjuvant chemotherapy within 9 months of diagnosis as well as those who did not. Patients were excluded if they had single or multiple diagnoses of colon cancer, stage I and IV rectal cancer, recurrent disease, incomplete study accession, only a baseline assessment available without further follow-up, lack of final staging, less than 9 months of follow-up, or lack of documentation of administration of neoadjuvant therapy and/or surgery.
Medical records were systematically reviewed at 4, 8, and 12 months and then yearly to document treatment and recurrence information. Baseline patient information included sociodemographic characteristics, insurance status, comorbidities (using Charlson index19), performance status, and household income. At the 4-month assessment, data were entered for clinical and pathologic TNM staging, histology, tumor location, distance from the anal verge, number of lymph nodes examined, number of lymph nodes involved with tumor, grade at diagnosis and primary surgery, presence/absence of lymphovascular invasion, perineural invasion, margin involvement (proximal, distal, radial), carcinoembryonic antigen level before and after surgery, and surgical procedures.
All cancer-directed treatments were collected during the follow-up assessments, including documented treatments delivered both at NCCN and outside institutions. The reasons for not receiving adjuvant chemotherapy were recorded, when available. Quality assurance included initial and follow-up training for the study personnel, online edit checking during web-based data entry, programmed logic checks against the pooled data repository, routine quality assurance reports to each institution, and onsite audits of a random sample of source documents against submitted data within the first few months of data collection (repeated annually).18 Informed consent or waiver of consent was approved by each center's institutional review board.
Statistical Analysis
Baseline characteristics were summarized as descriptive statistics (median and range for continuous variables, number and percentage for categorical variables), stratified by adjuvant versus no adjuvant therapy. Guideline concordance was defined as receipt of adjuvant chemotherapy within 9 months of diagnosis. The association between receipt of guideline-concordant adjuvant therapy and each parameter was assessed independently in a univariate logistic regression model. An independent variable was created for whether a patient had a complete response, was upstaged or downstaged, or had no change in stage; 63 patients (5%) were excluded from the logistic regression model because they were “unable to stage.“ Pathologic TNM was excluded from the multivariable model because of its colinearity with the clinical to pathologic downstaging variable, as was “lymph nodes positive,” which correlated with the pathologic TNM stage. Parameters found to be potentially associated with adjuvant therapy (P < .20) were included in the multivariable model, along with variables known to be associated with adjuvant therapy, clinical TNM stage and NCCN cancer center, as defined a priori. The final multivariable model included those predictors with a two-sided P value less than .05, along with the control variables defined a priori. Odds ratios (ORs) and associated 95% CIs were reported. To determine the reasons for not receiving adjuvant chemotherapy in accordance with American Society of Clinical Oncology/NCCN quality measures, the proportions of the patients who did not receive adjuvant therapy were calculated by reasons related to the patient (eg, patient declined treatment), physician (eg, physician recommended against treatment), and system level (eg, delayed treatment).
RESULTS
Description of the Study Cohort
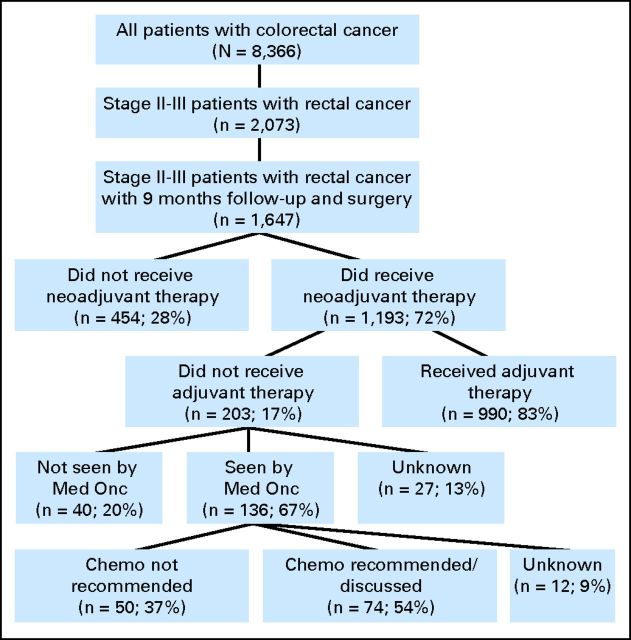
The charts of 8,366 patients with colorectal cancer, enrolled in the NCCN CRC Database at the eight NCCN institutions from September 1, 2005, through December 31, 2010, were reviewed (Fig 1). There were 2,073 patients with locally advanced rectal cancer, and of these, 1,647 patients had a minimum of 9 months of follow-up and primary surgery. Neoadjuvant therapy was administered to 1,193 patients, of whom 203 patients (17%) did not receive adjuvant chemotherapy.
Fig 1.
CONSORT diagram of patients with clinical stage II/III rectal cancer and administration of adjuvant chemotherapy. Chemo, chemotherapy; Med Onc, medical oncologist.
Table 1 describes the clinical and socioeconomic characteristics of the 1,193 patients who received neoadjuvant chemotherapy and radiation stratified by outcome.
Table 1.
Characteristics of Patients With Clinical Stage II/III and Locally Advanced Rectal Cancer and Received Neoadjuvant Therapy
| Characteristic | Adjuvant Therapy |
All Patients (N = 1,193) |
||||
|---|---|---|---|---|---|---|
| Yes (n = 990) |
No (n = 203) |
|||||
| No. | % | No. | % | No. | % | |
| Age at diagnosis, years | ||||||
| Median | 55 | 65 | 57 | |||
| Range | 22-93 | 21-89 | 21-93 | |||
| < 50 | 300 | 30 | 29 | 14 | 329 | 27 |
| 50-64 | 451 | 46 | 69 | 34 | 520 | 44 |
| 65-74 | 197 | 20 | 53 | 26 | 250 | 21 |
| 75+ | 42 | 4 | 52 | 26 | 94 | 8 |
| Sex | ||||||
| Male | 602 | 61 | 114 | 56 | 716 | 60 |
| Female | 388 | 39 | 89 | 44 | 477 | 40 |
| Racial/ethnic background | ||||||
| White Non-Hispanic | 789 | 80 | 166 | 82 | 955 | 80 |
| African American Non-Hispanic | 57 | 6 | 14 | 7 | 71 | 6 |
| Asian Non-Hispanic | 63 | 6 | 4 | 2 | 67 | 6 |
| Other Non-Hispanic | 7 | < 1 | 1 | < 1 | 8 | < 1 |
| Hispanic | 71 | 7 | 17 | 8 | 88 | 7 |
| Unknown | 3 | < 1 | 1 | < 1 | 4 | < 1 |
| Charlson comorbidity index | ||||||
| 0 | 760 | 77 | 123 | 61 | 883 | 74 |
| 1 | 154 | 15 | 50 | 25 | 204 | 17 |
| 2 | 55 | 6 | 16 | 8 | 71 | 6 |
| 3+ | 21 | 2 | 14 | 7 | 35 | 3 |
| ECOG performance status | ||||||
| 0 | 843 | 85 | 141 | 69 | 984 | 82 |
| 1 | 71 | 7 | 34 | 17 | 105 | 9 |
| 2+ | 15 | 2 | 7 | 3 | 22 | 2 |
| Unknown | 61 | 6 | 21 | 10 | 82 | 7 |
| Center | ||||||
| A | 45 | 5 | 17 | 8 | 62 | 5 |
| B | 85 | 9 | 12 | 6 | 97 | 8 |
| C | 48 | 5 | 9 | 4 | 57 | 5 |
| D | 298 | 30 | 30 | 15 | 328 | 27 |
| E | 43 | 4 | 17 | 8 | 60 | 5 |
| F | 69 | 7 | 50 | 25 | 119 | 10 |
| G | 338 | 34 | 56 | 28 | 394 | 33 |
| H | 64 | 6 | 12 | 6 | 76 | 6 |
| Insurance | ||||||
| Private | 652 | 66 | 74 | 36 | 726 | 61 |
| Medicare | 236 | 24 | 92 | 45 | 328 | 27 |
| Medicaid/Indigent | 59 | 6 | 27 | 13 | 86 | 7 |
| Other | 31 | 3 | 5 | 2 | 36 | 3 |
| Unknown | 12 | 1 | 5 | 2 | 17 | 1 |
| Household income | ||||||
| < 40K | 355 | 36 | 84 | 41 | 439 | 37 |
| 40 to < 60K | 304 | 31 | 63 | 31 | 367 | 31 |
| 60 to < 80K | 187 | 19 | 30 | 15 | 217 | 18 |
| ≥ 80K | 102 | 10 | 16 | 8 | 118 | 10 |
| Unknown | 42 | 4 | 10 | 5 | 52 | 4 |
| Primary site | ||||||
| Rectosigmoid junction* | 72 | 7 | 12 | 6 | 84 | 7 |
| Rectum, NOS | 918 | 93 | 191 | 94 | 1109 | 93 |
| Clinical TNM stage | ||||||
| Locally advanced† | 37 | 4 | 11 | 5 | 48 | 4 |
| II | 290 | 29 | 76 | 37 | 366 | 31 |
| III | 663 | 67 | 116 | 57 | 779 | 65 |
| Pathologic TNM stage | ||||||
| Locally advanced‡ | 12 | 1 | 8 | 4 | 20 | 2 |
| 0 | 190 | 19 | 51 | 25 | 241 | 20 |
| I | 254 | 26 | 52 | 26 | 306 | 26 |
| II | 232 | 23 | 47 | 23 | 279 | 23 |
| III | 302 | 31 | 45 | 22 | 347 | 29 |
| Clinical to pathologic stage: Was patient downstaged/upstaged?‡ | ||||||
| Complete responders (stage 0) | 190 | 19 | 51 | 25 | 241 | 20 |
| Downstaged (from stage II to I or from stage III to II) | 211 | 21 | 56 | 28 | 267 | 22 |
| Downstaged (from stage III to I) | 175 | 18 | 21 | 10 | 196 | 16 |
| Upstaged (from stage II to III) | 64 | 6 | 7 | 3 | 71 | 6 |
| No change in stage | 303 | 31 | 52 | 26 | 355 | 30 |
| Grade at diagnosis | ||||||
| 1 | 44 | 4 | 15 | 7 | 59 | 5 |
| 2 | 745 | 75 | 142 | 70 | 887 | 74 |
| 3 | 71 | 7 | 9 | 5 | 80 | 7 |
| Unknown | 110 | 11 | 34 | 17 | 144 | 12 |
| NA | 20 | 2 | 3 | 1 | 23 | 1 |
| Lymphovascular invasion at primary surgery | ||||||
| No | 642 | 65 | 119 | 59 | 761 | 64 |
| Yes | 140 | 14 | 28 | 14 | 168 | 14 |
| Unknown | 130 | 13 | 27 | 13 | 157 | 13 |
| NA | 78 | 8 | 29 | 14 | 107 | 9 |
| Perineural invasion at primary surgery | ||||||
| No | 518 | 52 | 109 | 54 | 627 | 53 |
| Yes | 107 | 11 | 18 | 9 | 125 | 10 |
| Unknown | 286 | 29 | 46 | 23 | 331 | 28 |
| NA | 80 | 8 | 30 | 15 | 110 | 9 |
| Any margins (proximal, distal or radial) | ||||||
| Negative | 714 | 72 | 125 | 62 | 839 | 70 |
| Positive/close | 69 | 7 | 21 | 10 | 90 | 8 |
| NA | 207 | 21 | 57 | 28 | 264 | 22 |
| Distance of tumor from anal verge, cm | ||||||
| < 6 | 356 | 36 | 87 | 43 | 443 | 37 |
| 6-8 | 304 | 31 | 42 | 21 | 346 | 29 |
| >8 | 275 | 28 | 56 | 28 | 331 | 28 |
| Unknown | 55 | 5 | 18 | 8 | 73 | 6 |
| CEA after surgery for patients with abnormal CEA at baseline§ | ||||||
| Normal | 167 | 17 | 31 | 15 | 198 | 17 |
| Abnormal | 32 | 3 | 10 | 5 | 42 | 4 |
| No CEA test after surgery | 24 | 2 | 15 | 7 | 39 | 3 |
| NA | 767 | 77 | 147 | 72 | 914 | 76 |
| Surgical procedure | ||||||
| LAR | 670 | 68 | 116 | 57 | 786 | 66 |
| APR | 209 | 21 | 65 | 32 | 274 | 23 |
| Total proctocolectomy | 24 | 2 | 8 | 4 | 32 | 3 |
| Total pelvic exenteration | 9 | 1 | 3 | 1 | 12 | 1 |
| Partial pelvic exenteration | 2 | < 1 | 1 | < 1 | 3 | < 1 |
| Proctectomy | 61 | 6 | 3 | 1 | 64 | 5 |
| Surgical complications | ||||||
| Anastomosis leak/peritonitis | 4 | < 1 | 6 | 3 | 10 | < 1 |
| Postoperative bleeding | 1 | < 1 | 0 | 0 | 1 | < 1 |
| Re-operation bowel obstruction | 3 | < 3 | 0 | 0 | 3 | < 1 |
| Re-operation wound infection | 35 | 4 | 24 | 12 | 59 | 5 |
| Other surgical procedures‖ | ||||||
| Ileostomy | 32 | 3 | 9 | 4 | 41 | 3 |
| Colostomy | 59 | 6 | 20 | 10 | 79 | 7 |
| Closure of ileostomy/colostomy | 587 | 59 | 76 | 37 | 663 | 56 |
| Endoscopic stenting | 9 | 1 | 6 | 3 | 15 | 1 |
| Surgical drainage of abscess | 9 | 1 | 6 | 3 | 15 | 1 |
NOTE. Patients presented to NCCN institutions between September 2005 and December 2010 and received neoadjuvant chemotherapy and pelvic radiation.
Abbreviations: APR, abdominoperineal resection; CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; LAR, low anterior resection; NA, not applicable; NOS, not otherwise specified.
Seventy-six (90%) treated as rectal, four (5%) treated as colon, four treated as indeterminate.
Locally advanced means no evidence of metastatic disease, but staging was not available. Patients who were not able to be staged clinically and pathologically were not included in this study.
Sixty-three of the patients were excluded from the downstaged/upstaged analyses, as these patients either had clinical TNM stage or a pathologic TNM stage of locally advanced, but stage of disease not further specified.
Baseline was defined as a CEA test before neoadjuvant therapy. NA (not applicable) represents patients who did not have abnormal CEA at baseline (n = 913 with normal CEA at baseline; n = 1 unknown if CEA test was done).
Represents procedures that were performed after the primary rectal surgery.
Univariate Analyses
Table 2 summarizes the association with adjuvant therapy for available clinical and socioeconomic variables. It includes only the variables that were significantly associated with administration of adjuvant therapy from the univariate logistic regression and remained significant in the multivariable logistic regression model (P < .05).
Table 2.
Factors Associated With Receiving Adjuvant Therapy for Patients With Stage II/III and Locally Advanced Rectal Cancer
| Variable | Patients With Adjuvant Therapy |
Unadjusted OR* | 95% CI | P* | Adjusted OR† | 95% CI | P† | |
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Age at diagnosis, years | ||||||||
| < 50 | 300 | 91 | Referent | < .001 | Referent | < .001 | ||
| 50-64 | 451 | 87 | 0.63 | 0.40 to 0.99 | 0.55 | 0.32 to 0.94 | ||
| 65-74 | 197 | 79 | 0.36 | 0.22 to 0.59 | 0.22 | 0.10 to 0.47 | ||
| 75+ | 42 | 45 | 0.08 | 0.05 to 0.14 | 0.04 | 0.02 to 0.10 | ||
| ECOG performance status | ||||||||
| 0 | 843 | 86 | Referent | < .001 | Referent | .002 | ||
| 1+ | 86 | 68 | 0.35 | 0.23 to 0.53 | 0.40 | 0.24 to 0.68 | ||
| Unknown | 61 | 74 | 0.49 | 0.29 to 0.82 | 0.59 | 0.29 to 1.16 | ||
| Center | ||||||||
| A | 45 | 73 | 0.44 | 0.24 to 0.82 | < .001 | 0.35 | 0.17 to 0.73 | < .001 |
| B | 85 | 88 | 1.17 | 0.60 to 2.29 | 1.35 | 0.61 to 2.98 | ||
| C | 48 | 84 | 0.88 | 0.41 to 1.90 | 2.19 | 0.79 to 6.06 | ||
| D | 298 | 91 | 1.65 | 1.03 to 2.63 | 1.38 | 0.81 to 2.36 | ||
| E | 43 | 72 | 0.42 | 0.22 to 0.79 | 0.65 | 0.30 to 1.41 | ||
| F | 69 | 58 | 0.23 | 0.14 to 0.36 | 0.27 | 0.15 to 0.48 | ||
| G | 338 | 86 | Referent | Referent | ||||
| H | 64 | 84 | 0.88 | 0.45 to 1.74 | 0.86 | 0.39 to 1.89 | ||
| Insurance | ||||||||
| Private | 652 | 90 | Referent | < .001 | Referent | .009 | ||
| Medicare | 236 | 72 | 0.29 | 0.21 to 0.41 | 1.42 | 0.72 to 2.80 | ||
| Medicaid/indigent | 59 | 69 | 0.25 | 0.15 to 0.42 | 0.35 | 0.19 to 0.67 | ||
| Other | 31 | 86 | 0.70 | 0.27 to 1.87 | 0.74 | 0.26 to 2.14 | ||
| Unknown | 12 | 71 | 0.27 | 0.09 to 0.80 | 0.90 | 0.24 to 3.43 | ||
| Clinical TNM stage | ||||||||
| Locally advanced | 37 | 77 | 0.59 | 0.29 to 1.19 | .03 | 0.30 | 0.04 to 2.45 | .26 |
| II | 290 | 79 | 0.67 | 0.49 to 0.92 | 0.77 | 0.51 to 1.14 | ||
| III | 663 | 85 | Referent | Referent | ||||
| Clinical to pathologic TNM stage: was the patient downstaged or upstaged?‡ | ||||||||
| Complete responders§ | 190 | 79 | 0.64 | 0.42 to 0.98 | .08 | 0.62 | 0.37 to 0.99 | .03 |
| Downstaged | 386 | 83 | 0.86 | 0.59 to 1.26 | 0.84 | 0.54 to 1.31 | ||
| Upstaged | 64 | 90 | 1.57 | 0.68 to 3.61 | 2.54 | 0.95 to 6.80 | ||
| No Change in stage | 303 | 31 | Referent | Referent | ||||
| Re-operation wound infection | ||||||||
| No | 955 | 84 | Referent | < .001 | Referent | < .001 | ||
| Yes | 35 | 59 | 0.27 | 0.16 to 0.47 | 0.25 | 0.13 to 0.48 | ||
| Closure of ileostomy/colostomy | ||||||||
| No | 403 | 76 | 0.41 | 0.30 to 0.56 | < .001 | 0.60 | 0.40 to 0.89 | .01 |
| Yes | 587 | 89 | Referent | Referent | ||||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; OR, odds ratio.
Unadjusted OR = univariate logistic regression.
Adjusted OR = multivariable logistic regression. The final multivariable logistic regression model has the following factors: age at diagnosis, ECOG performance status, center, insurance, clinical TNM stage, downstaged/upstaged, re-operation of wound infection, and closure of ileostomy/colostomy. Factors that were not significant in the univariate and/or multivariable model were not reported (sex, race/ethnic background, income, histology, grade, Charlson comorbidity score, pathologic TNM stage, lymphovascular invasion, perineural invasion, margin status, distance from and verge, carcinoembryonic antigen, lower anterior resection, abdominoperineal resection, proctectomy, and colostomy).
Sixty-three of the patients were excluded from the downstaged/upstaged analyses, as these patients either had clinical TNM stage or a pathologic TNM stage of locally advanced, but stage of disease not further specified.
Complete responders are patients with pathologic TNM stage 0.
Multivariable Analyses
Table 2 also summarizes the multivariable association with adjuvant therapy, with the following factors found to be statistically significant: age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), NCCN cancer center, insurance, clinical to pathologic downstaging, re-operation of a wound infection, and closure of an ileostomy/colostomy. Clinical TNM stage was not significant in the multivariable model, but was forced in the final model as a control variable.
Patients ≥ 50 years of age (v < 50 years) at diagnosis were less likely to receive adjuvant chemotherapy: age 50 to 64 years (adjusted OR = 0.55, 95% CI, 0.32 to 0.94), age 65 to 74 years (adjusted OR = 0.22, 95% CI, 0.10 to 0.47), age ≥ 75 years (adjusted OR = 0.04, 95% CI, 0.02 to 0.10). Those with ECOG PS ≥ 1 (v ECOG PS = 0) were less likely to receive adjuvant chemotherapy (adjusted OR = 0.40, 95% CI, 0.24 to 0.68). Patients at Center A (OR = 0.35, 95% CI, 0.17 to 0.73) and Center F (OR = 0.27, 95% CI, 0.15 to 0.48) were less likely to receive adjuvant chemotherapy than patients at Center G, which was chosen as the referent group because it had the largest number of patients. Compared with private insurance, Medicaid and indigent patients were less likely to receive adjuvant chemotherapy (adjusted OR = 0.35, 95% CI, 0.19 to 0.67). Patients with a complete response were less likely to receive adjuvant chemotherapy (adjusted OR = 0.62, 95% CI, 0.37 to 0.99) compared with those with no change in cTNM to pTNM. Patients who had a re-operation of a wound infection were less likely to receive adjuvant chemotherapy (adjusted OR = 0.25, 95% CI, 0.13 to 0.48) compared with those who had no re-operation, as were patients without closure of an ileostomy/colostomy (adjusted OR = 0.60, 95% CI, 0.40 to 0.89) compared with those with closure.
Among the 203 patients who did not receive adjuvant chemotherapy, 67% were seen by a medical oncologist postoperatively, 20% were not seen by a medical oncologist, and for 13%, it was unknown whether they were seen by a medical oncologist (Fig 1). Within the patient cohort seen by a medical oncologist (n = 136), 54% had a discussion with their physician regarding further recommendations about adjuvant chemotherapy, 37% had documentation that adjuvant chemotherapy was not recommended, and 9% had no chart documentation regarding whether or not treatment was recommended. For these patients, reasons cited for not receiving adjuvant therapy recommendation included presence of comorbid illnesses alone (n = 22), therapy not indicated (n = 20), comorbid illnesses/older age (n = 3), disease recurrence (n = 2), death (n = 1), and unknown reasons (n = 2). The reasons chemotherapy was not administered despite physician recommendations included patients declined treatment (n = 54), recurrence before treatment administration (n = 8), no treatment documented at 12-month assessment (n = 3), patient death (n = 2), patient transferred to other center (n = 2), and reason unknown (n = 5). The database does not include the specific reasons why patients declined treatment. Table 3 summarizes the 990 patients who started adjuvant chemotherapy and the frequency and the reason the adjuvant therapy was discontinued (n = 290, 29%).
Table 3.
Reasons Adjuvant Chemotherapy Ended
| Reason | No. of Patients | % |
|---|---|---|
| Completed adjuvant treatment | 700 | 70.71 |
| Toxicity | 160 | 16.16 |
| Patient/family preference | 40 | 4.04 |
| Unknown reason | 39 | 3.94 |
| Reason not entered | 29 | 2.93 |
| Other reason | 8 | 0.81 |
| Cancer progression | 8 | 0.81 |
| Patient died | 3 | 0.30 |
| Insurance issues | 2 | 0.20 |
| Transferred care | 1 | 0.10 |
DISCUSSION
Although decreased local recurrence rates for patients with stage II/III rectal cancer have been achieved with the use of combination chemotherapy and radiation therapy, 35% of patients nevertheless develop metastatic disease.2,20 Current NCCN CRC Guidelines recommend completion of a 6-month course of adjuvant chemotherapy for patients with stage II/III rectal cancer; however, the rate of administration of adjuvant chemotherapy at NCCN institutions has been variable.18,21 The goals of the current study were both to evaluate the reasons why adjuvant chemotherapy is not delivered and explore the relationship between patient characteristics and administration of adjuvant chemotherapy.
Our analysis found that the most common reasons adjuvant chemotherapy was not recommended by a medical oncologist was secondary to comorbid illness (25 of 50, 50%) and recommended but not received was patient refusal (54 of 74, 73%). Similar findings have been noted in other colorectal cancer studies in which decreased administration of adjuvant chemotherapy was due to patient refusal, presence of comorbid conditions, and lack of clinical indication by the physician.8–10 A population-based cohort of patients with both stage II/III rectal cancer and stage III colon cancer from the California Cancer Registry demonstrated that the principal reasons for not receiving adjuvant chemotherapy differed by patient age.9 Among patients ≥ 85 years of age, comorbidities and advanced age were cited as primary reasons. For those offered postoperative therapy, age remained the strongest predictor of patient refusal, with lower rates for younger patients and reaching almost 50% in the group ≥ 85 years of age. Despite the increasing number of people older than 75 years, the use of colorectal cancer adjuvant therapy in this age group is declining11,12 and is an underrepresented population in clinical trials.13 Some have noted worse outcomes in an aged population with comorbid conditions.22–25 Other studies have demonstrated that elderly individuals with a good performance status tolerate chemotherapy just as well as the younger population,26,27 with no significant interactions between age and treatment efficacy.27–29
In contrast to the NCCN study in which 83% of patients received recommended adjuvant therapy, a cross-sectional study from the Veteran's Medical Center in Houston found that only 42.5% of patients with stage II/III rectal cancer received recommended therapy (defined as pre- or postoperative radiation therapy, surgical resection, and postoperative chemotherapy).8 Among 57.5% of the eligible patients who did not receive recommended therapy, 36% had comorbidities, 18% were believed not to require therapy by the physicians, 31% died before follow-up or had postoperative complications, and 15% declined therapy despite physician recommendations.
Physicians are concerned with increased therapy-induced toxicity rates and inferior survival for those with comorbidities.30 A retrospective review of the medical records from the National Institute on Aging, National Cancer Institute, and National Cancer Institute Surveillance, Epidemiology, and End Results tumor registry documented preexisting conditions in the elderly with colon cancer and evaluated the effects of comorbidity on early mortality, stratified by various degrees of Life Threat Risk (High Impact, Moderate, Low, and Negligible).25 Early mortality was significantly associated with higher stage of disease and the total number of comorbid and chronic conditions in the High Impact Life Threat category. Although this correlation is important, the patients in the NCCN study were well enough to receive neoadjuvant chemoradiotherapy and surgery and had an ECOG PS of 0 or 1 (82% and 9%, respectively). Tolerance of previous preoperative therapy and surgery questions the role of comorbidity and failure to treat with adjuvant chemotherapy.
Of those patients who did not receive adjuvant therapy, it was unknown whether 27 patients (13%) were seen by a medical oncologist. Of those seen by a medical oncologist, 12 patients (9%) did not have documentation regarding whether chemotherapy was or was not recommended. Several studies attribute missing data because of lack of available information, data entry errors, and working under time pressure paired with low physician job satisfaction.31–34 Difficulty with documentation in the NCCN study may be related to the variability in the numbers of patients who receive all medical care at the same institution. The current NCCN database only contains therapy that occurs outside the NCCN institution if the information is noted in the NCCN chart, thus limiting an accurate assessment of whether postoperative treatment was given or not.
Various studies have assessed the association between cancer care and insurance, but few have investigated this relationship in colorectal cancer. Limited reports have shown that medical practices and patient outcomes were not affected by an insurance plan, although patients with low incomes fared worse when enrolled in a health maintenance organization (HMO) compared with a fee-for-service (FFS) plan.35–37 In the colorectal cancer population, there was no difference in the rates of definitive surgery, chemotherapy, and radiation therapy as well as survival in patients with HMO plans versus FFS plans.38,39 In contrast, Roetzheim et al40 found that colorectal cancer treatments in the state of Florida varied considerably based on the insurance coverage, including those with commercial HMO, who were less likely to receive chemotherapy and had greater mortality than those with FFS insurance. Our data showed that patients with Medicaid and indigent patients are less likely to receive adjuvant chemotherapy.
Clinical to pathologic downstaging after neoadjuvant chemoradiotherapy was associated with decreased administration of adjuvant chemotherapy. Although the addition of chemotherapy to radiation has demonstrated improved locoregional control and increased likelihood of pathologic complete response, this benefit has not been translated to improved overall survival.2,20,41,42 In contrast, a subgroup analysis from the European Organisation for Research and Treatment of Cancer 22921 study has shown that patients downstaged to ypT0-2 disease benefited from adjuvant chemotherapy, whereas those with residual ypT3-4 disease did not.4–6 Other retrospective reviews also have shown that patients who respond to neoadjuvant therapy are the most likely to achieve enhanced efficacy from adjuvant chemotherapy.5,6 Currently, there are no data from prospective randomized clinical trials to define the optimal use of postoperative chemotherapy, including the degree of improved outcome for those with tumor downstaging or a pCR after neoadjuvant treatment.3–6,43–50 Although not definitive, the data demonstrating the correlation between neoadjuvant therapy response and benefit from adjuvant chemotherapy warrants routine postoperative discussion among the surgeon, medical oncologist, and patient.
Re-operation, wound infection, and lack of closure of ileostomy/colostomy sites were also associated with decreased administration of adjuvant chemotherapy. In stage III colon cancer patients, Hershman et al51 demonstrated that delayed adjuvant chemotherapy administration greater than 3 months was associated with higher mortality rates.51 Factors associated with delays included older age, increased comorbidities, tumor grade, and marital status. Cheung et al52 conducted a study to determine the rates and causes of adjuvant chemotherapy delays and the effects of delay on the outcome in patients with stage II/III rectal cancer. They found that patients discharged from the hospital more than 4 weeks postoperatively or readmitted to the hospital more than once waited 3 or more months before receiving their first cycle of therapy, resulting in a significantly worse median survival.
Although this large NCCN database provides the characteristics of patients who do and do not receive recommended adjuvant chemotherapy for rectal cancer and reasons why patients do not receive adjuvant chemotherapy or do not complete the recommended/planned therapy, this analysis does have limitations. These data represent patients who were seen at academic comprehensive cancer centers and thus may not reflect the true percentage of patients with rectal cancer across the United States who do not receive adjuvant therapy, or the full variability of reasons why therapy was not administered or reasons why patients who initiated adjuvant therapy did not complete the treatment course. In addition, some of the individuals received a component of their care at a non-NCCN institution, resulting in under-reporting of information in the medical charts, thus potentially limiting the overall assessment of what occurred in the postoperative period. In addition, 20% of those who did not receive adjuvant therapy were not seen by a medical oncologist, an observation that warrants further investigation but cannot be elucidated from information available in the database. Furthermore, this analysis did not include recurrence or survival data, which will be important for future assessment to measure the true outcome effect for patients who do not receive adjuvant therapy or receive incomplete adjuvant treatment. In addition, the benefit of adjuvant chemotherapy after neoadjuvant chemoradiotherapy and surgery in locally advanced rectal cancer is not definitive and, therefore, may be a reason for the variability in patient selection for adjuvant chemotherapy among institutions. There is no conclusive evidence to define the optimal adjuvant chemotherapy regimen or the most optimal subgroups of patients to be treated, which may lead to variability in physician recommendations. Prospective clinical trials will be required to provide the evidence that defines systemic treatment strategies, including a focus on tumor biology, to enhance patient selection for treatment and to improve survival for patients with stage II/III rectal cancer.
Footnotes
Presented at the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joyce C. Niland, Deborah Schrag, Al B. Benson III
Administrative support: Polina Khrizman, Joyce C. Niland, Dana Milne, Al B. Benson III
Provision of study materials or patients: Kelli Bullard Dunn, Paul F. Engstrom, Stephen Shibata, John M. Skibber, Martin R. Weiser, Deborah Schrag, Al B. Benson III
Collection and assembly of data: Anna ter Veer, Dana Milne, Kelli Bullard Dunn, Paul F. Engstrom, Stephen Shibata, John M. Skibber, Martin R. Weiser, Deborah Schrag, Al B. Benson III
Data analysis and interpretation: Polina Khrizman, Dana Milne, William E. Carson III, Deborah Schrag, Al B. Benson III
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A Siegel R Ward E, etal: Cancer statistics, 2009 CA Cancer J Clin 59:225–249,2009 [DOI] [PubMed] [Google Scholar]
- 2.Bosset JF Collette L Calais G, etal: Chemotherapy with preoperative radiotherapy in rectal cancer N Engl J Med 355:1114–1123,2006 [DOI] [PubMed] [Google Scholar]
- 3.Chan AK Wong AO Langevin J, etal: Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: A phase II dose escalation study Int J Radiat Oncol Biol Phys 48:843–856,2000 [DOI] [PubMed] [Google Scholar]
- 4.Collette L Bosset JF den Dulk M, etal: Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group J Clin Oncol 25:4379–4386,2007 [DOI] [PubMed] [Google Scholar]
- 5.Das P Skibber JM Rodriguez-Bigas MA, etal: Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer Am J Clin Oncol 29:219–224,2006 [DOI] [PubMed] [Google Scholar]
- 6.Janjan NA Crane C Feig BW, etal: Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer Am J Clin Oncol 24:107–112,2001 [DOI] [PubMed] [Google Scholar]
- 7.Fietkau R Barten M Klautke G, etal: Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer Dis Colon Rectum 49:1284–1292,2006 [DOI] [PubMed] [Google Scholar]
- 8.Abraham NS Gossey JT Davila JA, etal: Receipt of recommended therapy by patients with advanced colorectal cancer Am J Gastroenterol 101:1320–1328,2006 [DOI] [PubMed] [Google Scholar]
- 9.Ayanian JZ Zaslavsky AM Fuchs CS, etal: Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort J Clin Oncol 21:1293–1300,2003 [DOI] [PubMed] [Google Scholar]
- 10.Mahoney T Kuo YH Topilow A, etal: Stage III colon cancers: Why adjuvant chemotherapy is not offered to elderly patients Arch Surg 135:182–185,2000 [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL Harlan LC Kaplan RS, etal: Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer J Clin Oncol 20:1192–1202,2002 [DOI] [PubMed] [Google Scholar]
- 12.Schrag D Cramer LD Bach PB, etal: Age and adjuvant chemotherapy use after surgery for stage III colon cancer J Natl Cancer Inst 93:850–857,2001 [DOI] [PubMed] [Google Scholar]
- 13.Trimble EL Carter CL Cain D, etal: Representation of older patients in cancer treatment trials Cancer 74:2208–2214,1994 [DOI] [PubMed] [Google Scholar]
- 14.Park IJ You YN Agarwal A, etal: Neoadjuvant treatment response as an early response indicator for patients with rectal cancer J Clin Oncol 30:1770–1776,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das P Skibber JM Rodriguez-Bigas MA, etal: Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer Cancer 109:1750–1755,2007 [DOI] [PubMed] [Google Scholar]
- 16.Silberfein EJ Kattepogu KM Hu CY, etal: Long-term survival and recurrence outcomes following surgery for distal rectal cancer Ann Surg Oncol 17:2863–2869,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KD Tan D Das P, etal: Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation Ann Surg 251:261–264,2010 [DOI] [PubMed] [Google Scholar]
- 18.Romanus D Weiser MR Skibber JM, etal: Concordance with NCCN Colorectal Cancer Guidelines and ASCO/NCCN Quality Measures: An NCCN institutional analysis J Natl Compr Canc Netw 7:895–904,2009 [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME Pompei P Ales KL, etal: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation J Chronic Dis 40:373–383,1987 [DOI] [PubMed] [Google Scholar]
- 20.Sauer R Becker H Hohenberger W, etal: Preoperative versus postoperative chemoradiotherapy for rectal cancer N Engl J Med 351:1731–1740,2004 [DOI] [PubMed] [Google Scholar]
- 21.Benson AB 3rd Arnoletti JP Bekaii-Saah T, etal: NCCN Guidelines Version 3.2012 www.nccn.org [Google Scholar]
- 22.Dale DC: Poor prognosis in elderly patients with cancer: The role of bias and undertreatment J Support Oncol 1:11–17,2003 [PubMed] [Google Scholar]
- 23.Extermann M: Measurement and impact of comorbidity in older cancer patients Crit Rev Oncol Hematol 35:181–200,2000 [DOI] [PubMed] [Google Scholar]
- 24.Repetto L: Greater risks of chemotherapy toxicity in elderly patients with cancer J Support Oncol 1:18–24,2003 [PubMed] [Google Scholar]
- 25.Yancik R Wesley MN Ries LA, etal: Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study Cancer 82:2123–2134,1998 [PubMed] [Google Scholar]
- 26.Popescu RA Norman A Ross PJ, etal: Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older J Clin Oncol 17:2412–2418,1999 [DOI] [PubMed] [Google Scholar]
- 27.Sargent DJ Goldberg RM Jacobson SD, etal: A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients N Engl J Med 345:1091–1097,2001 [DOI] [PubMed] [Google Scholar]
- 28.Fata F Mirza A Craig G, etal: Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: A 10-year experience of the Geisinger Medical Center Cancer 94:1931–1938,2002 [DOI] [PubMed] [Google Scholar]
- 29.Iwashyna TJ, Lamont EB: Effectiveness of adjuvant fluorouracil in clinical practice: A population-based cohort study of elderly patients with stage III colon cancer J Clin Oncol 20:3992–3998,2002 [DOI] [PubMed] [Google Scholar]
- 30.Lee L Cheung WY Atkinson E, etal: Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review J Clin Oncol 29:106–117,2011 [DOI] [PubMed] [Google Scholar]
- 31.Ammenwerth E, Spötl HP: The time needed for clinical documentation versus direct patient care. A work-sampling analysis of physicians' activities Methods Inf Med 48:84–91,2009 [PubMed] [Google Scholar]
- 32.Chan KS, Fowles JB, Weiner JP: Review: Electronic health records and the reliability and validity of quality measures: A review of the literature Med Care Res Rev 67:503–527,2010 [DOI] [PubMed] [Google Scholar]
- 33.Herzberg S Rahbar K Stegger L, etal: Concept and implementation of a computer-based reminder system to increase completeness in clinical documentation Int J Med Inform 80:351–358,2011 [DOI] [PubMed] [Google Scholar]
- 34.Hogan WR, Wagner MM: Accuracy of data in computer-based patient records J Am Med Inform Assoc 4:342–355,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenfield S Rogers W Mangotich M, etal: Outcomes of patients with hypertension and non-insulin dependent diabetes mellitus treated by different systems and specialties: Results from the medical outcomes study JAMA 274:1436–1444,1995 [PubMed] [Google Scholar]
- 36.Hellinger FJ: The effect of managed care on quality: A review of recent evidence Arch Intern Med 158:833–841,1998 [DOI] [PubMed] [Google Scholar]
- 37.Ware JE Jr Bayliss MS Rogers WH, etal: Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems: Results from the Medical Outcomes Study JAMA 276:1039–1047,1996 [PubMed] [Google Scholar]
- 38.Francis AM, Polissar L, Lorenz AB: Care of patients with colorectal cancer: A comparison of a health maintenance organization and fee-for-service practices Med Care 22:418–429,1984 [DOI] [PubMed] [Google Scholar]
- 39.Vernon SW Hughes JI Heckel VM, etal: Quality of care for colorectal cancer in a fee-for-service and health maintenance organization practice Cancer 69:2418–2425,1992 [DOI] [PubMed] [Google Scholar]
- 40.Roetzheim RG Pal N Gonzalez EC, etal: Effects of health insurance and race on colorectal cancer treatments and outcomes Am J Public Health 90:1746–1754,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceelen WP, Van Nieuwenhove Y, Fierens K: Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer Cochrane Database Syst Rev CD006041 2009 [DOI] [PubMed] [Google Scholar]
- 42.Gérard JP Conroy T Bonnetain F, etal: Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203 J Clin Oncol 24:4620–4625,2006 [DOI] [PubMed] [Google Scholar]
- 43.Cionini L Manfredi B Sainato A, etal: Randomized study of postoperative chemotherapy (CT) after preoperative chemoradiation (CTRT) in locally advanced rectal cancer (LARC): Preliminary results Eur J Cancer 37:S300,2001 [Google Scholar]
- 44.Li RL: Combination of surgery, radiotherapy and chemotherapy for rectal cancer: A 423 cases report [in Chinese] Zhonghua Zhong Liu Za Zhi 14:213–215,1992 [PubMed] [Google Scholar]
- 45.Vergo M, Nimeiri H, Benson AB, 3rd: Adjuvant chemotherapy after neoadjuvant chemoradiation and surgery: A quest to improve survival for stage II and III rectal cancer Curr Colorectal Cancer Rep 5:151–157,2009 [Google Scholar]
- 46.Schroen AT, Cress RD: Use of surgical procedures and adjuvant therapy in rectal cancer treatment: A population-based study Ann Surg 234:641–651,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cronin DP Harlan LC Potosky AL, etal: Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer Am J Gastroenterol 101:2308–2318,2006 [DOI] [PubMed] [Google Scholar]
- 48.Cree M Tonita J Turner D, etal: Comparison of treatment received versus long-standing guidelines for stage III colon and stage II/III rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004 Clin Colorectal Cancer 8:141–145,2009 [DOI] [PubMed] [Google Scholar]
- 49.Pisu M Richardson LC Kim YI, etal: Less-than-standard treatment in rectal cancer patients: Which patients are at risk? J Natl Med Assoc 102:190–198,2010 [DOI] [PubMed] [Google Scholar]
- 50.Schrag D Gelfand SE Bach PB, etal: Who gets adjuvant treatment for stage II and III rectal cancer? Insight from Surveillance, Epidemiology, and End Results–Medicare J Clin Oncol 19:3712–3718,2001 [DOI] [PubMed] [Google Scholar]
- 51.Hershman D Hall MJ Wang X, etal: Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer Cancer 107:2581–2588,2006 [DOI] [PubMed] [Google Scholar]
- 52.Cheung WY, Neville BA, Earle CC: Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer Dis Colon Rectum 52:1054–1063,2009 [DOI] [PubMed] [Google Scholar]