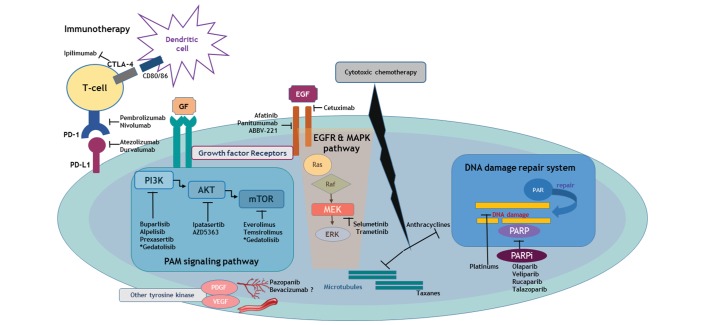
Figure 1.
Signalling pathways and involved entities that are unravelling experimental therapeutic targets for TNBC. Depicted molecular landscape of TNBC confers an insight of novel and investigational targeted therapeutic strategy which are directly unlocking its heterogeneous biology. In the context of its intrinsic genetic instability which derives an immunogenic microenvironment, blockade of the immune-checkpoint targeting PD-1 and PD-L1 as well as CTLA-4 can boost the adaptive immune reaction. PAM signalling pathways are actively participating in cell cycle regulation, which are in the tight network with various growth factors including EGF and MAPK signalling. Platinum-based agents and PARPi is a master regulator of DNA damage repair and can induce synergistic inhibitory effect in TNBC harbouring BRCAness. Other multikinase inhibitors involving angiogenesis or developmental process are also a potential therapeutic entity of current interest. All these investigational but key targets are consistently interacting with cytotoxic effect of conventional chemotherapy. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular signal-related kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; PAM, PI3K-Akt-mTOR; PARP, poly(ADP-ribose) polymerase; PARPi, PARP inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TNBC, triple-negative breast cancer.