Abstract
Background
Regulatory agencies have concluded that sodiumglucose cotransporter 2 (SGLT2) inhibitors lead to ketoacidosis, but published literature on this point remains controversial.
Methods
We searched the FDA Adverse Event Reporting System (FAERS) for reports of acidosis in patients treated with canagliflozin, dapagliflozin, or empagliflozin (from the date of each drug’s FDA approval until May 15, 2015). We compared the number of SGLT2 inhibitor-related reports to reports of acidosis in patients treated with the 2 most commonly used DPP4 inhibitors: sitagliptin and saxagliptin. We estimated relative risks of acidosis by relating the number of reports to cumulative drug sales (a surrogate for patient exposure).
Results
FAERS contained 259 reports of acidosis (including 192 reports of ketoacidosis) for SGLT2 inhibitors compared with 477 reports of acidosis for DPP4 inhibitors (including 71 reports of ketoacidosis). Based on estimated patient exposure, the overall risk of developing acidosis was ~14-fold higher for SGLT2 inhibitors. Among 51 SGLT2 inhibitor-related reports with quantifiable metabolic information, 20 cases occurred in patients with type 1 diabetes (T1D), 25 in type 2 diabetes (T2D), and 6 in patients with unspecified type of diabetes. After excluding patients withT1D and focusing on patients identified as having T2D, we estimate that SGLT2 inhibitors were associated with ~7-fold increase in developing acidosis. Seventy-one percent had euglycemic ketoacidosis.
Conclusions
Our results support the FDA’s warning that SGLT2 inhibitors lead to ketoacidosis, as evidenced by an increased reporting rate for acidosis above that in a comparator population treated with DPP4 inhibitors.
Keywords: adverse event, FAERS, ketoacidosis, DKA, SGLT2 inhibitors
1 | INTRODUCTION
Sodium glucose cotransporter 2 (SGLT2) inhibitors are the newest class of oral anti-diabetic medications. Empagliflozin and canagliflozin, 2 members of the SGLT2 inhibitor class, have been reported to decrease the risk of major adverse cardiovascular events.1,2 Preliminary data also suggests that these 2 drugs may slow the progression of diabetic kidney disease.2,3 Cardiovascular outcome studies are currently underway for dapagliflozin.4 During the time that SGLT2 inhibitors have been marketed in the United States, the FDA has identified several adverse effects of these drugs. The FDA approved Prescribing Information states that canagliflozin causes loss of bone mineral density and increases the risk of bone fractures and amputations.5 Data supporting these side effects have been reported in the peer-reviewed literature.2,6,7 While the Prescribing Information for the 3 marketed SGLT2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin) cites an increase in the risk of ketoacidosis, the detailed data supporting these statements from the FDA have not yet been published in the peer-reviewed literature. Nevertheless, a randomized double-blind study of off-label use in type 1 diabetes (T1D) has provided convincing evidence that canagliflozin increases the risk of ketone-related adverse events from 0% (placebo) to 9.4% (canagliflozin, 300 mg).8 Based on published data, the risk appears to be smaller when these drugs are used for the approved indication of glycemic control in type 2 diabetic patients. Utilizing the database for 17 596 patients in randomized drug development studies, Erondu et al9 reported that canagliflozin increased the risk of DKA and related events from 0.03% (comparator drugs) to 0.11% (canagliflozin, 300 mg). The EMPA-REG OUTCOME study included 7020 patients. Only 1 placebo-treated patient (0.04%) was reported to develop diabetic ketoacidosis in contrast to 4 empagliflozin-treated patients (0.09%). Although it is impossible to draw firm conclusions based on the small number of cases of ketoacidosis in these studies, published data from randomized controlled clinical trials are consistent with the hypothesis that both SGLT2 inhibitors cause a 2-fold to 4-fold increase in the risk of ketoacidosis. A meta-analysis with data from the 3 large cardiovascular outcome studies (including dapagliflozin (NCT01730534) which is projected to enrol 17 150 and CANVAS which enrolled 4330 patients) may provide sufficient statistical power to estimate the risk of drug-associated ketoacidosis. In the meantime, in the absence of appropriately sized randomized controlled trials, physicians and patients are forced to look elsewhere for evidence to assess the risk of SGLT2 inhibitor-associated ketoacidosis.
The FDA warning about the risk of ketoacidosis (May, 2015) cited 20 cases of acidosis in SGLT2 inhibitor-treated patients identified in the FDA Adverse Event Reporting System (FAERS) database, followed by another warning in December 2015 citing 73 cases.10,11 Subsequently, in June, 2016, the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) published a position paper that raised questions about the FDA’s conclusion12: “The incidence of DKA in T2D treated with SGLT-2 inhibitors does not appear to exceed the low levels occurring in the general diabetes population.” In other words, more than a year after FDA issued the first warning that SGLT2 inhibitors increase the risk of ketoacidosis, 2 leading professional organizations of clinical endocrinologists questioned the basic premise that the incidence of ketoacidosis is increased among patients taking this class of drugs. We conducted the present study to address concerns expressed in the AACE/ACE position statement—for example, by comparing rates of ketoacidosis in SGLT2 inhibitor-treated patients to a comparator group (ie, patients treated DPP4 inhibitors). Our analysis is entirely consistent with and supports the FDA’s conclusion the there is a greater incidence of reports of acidosis among patients treated with SGLT2 inhibitors as compared with the incidence observed in a comparator group. When we focused on type 2 diabetic patients and excluded patients with the diagnosis of T1D, we estimate that the risk of acidosis is increased approximately 7-fold in type 2 diabetic patients treated with SGLT2 inhibitors.
While this paper was under review, several recent publications addressed the question of whether SGLT2 inhibitors increase the risk of ketoacidosis. Based on an analysis of a Danish database, Jensen et al13 reported that SGLT2 inhibitors were not associated with a significant increase in the risk of ketoacidosis. By contrast, based on an analysis of a US claims database describing experience in commercially insured US patients (Truven MarketScan), Fralick et al14 reported that SGLT2 inhibitors were associated with a twofold increase in the risk of ketoacidosis as compared with patients treated with DPP4 inhibitors. Fadini et al15 published an analysis of data from the FAERS database, but did not compare the reporting rate for ketoacidosis in SGLT2 inhibitors to the baseline rate in comparable patients not taking an SGLT2 inhibitor.
2 | METHODS
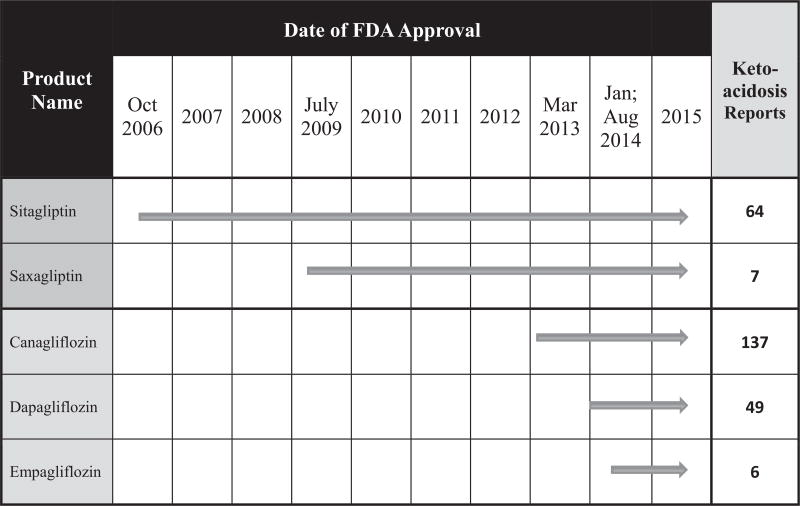
FDA provided us with FAERS reports associated with SGLT2 inhibitors received between the respective dates of FDA approval through May, 2015 (Table 1; search terms and details of FAERS search available in Online Supplement 1). We compared these reports with the 2 most widely prescribed DPP4 inhibitors (sitagliptin and saxagliptin) subsequent to their respective dates of approval. SGLT2 inhibitor-related reports including quantifiable information on metabolic derangements consistent with ketoacidosis (pH, ketonuria or ketonemia, bicarbonate, acid base disturbance) were included in a subanalysis. We applied FDA’s definition of euglycemic diabetic ketoacidosis (ie, plasma glucose ≤250 mg/dL).16 In our analysis of DPP4 inhibitors, we focused exclusively on sitagliptin and saxagliptin, which represented 89% and 8% of DPP4 inhibitor sales, respectively, in years leading up to May, 2015. Linagliptin (representing only 2% of sales) is often the first choice DPP4 inhibitor for patients with chronic kidney disease, a population with particularly severe disease who might be expected to experience above average rates of treatment-emergent adverse events. Alogliptin (representing only ~0.3% of sales) was approved in Japan substantially before approval in the United States. Because of the possibility that physicians in different countries might have different practices with respect to reporting adverse events to the FAERS database, we excluded alogliptin-associated reports from our analysis. By focusing on sitagliptin and saxagliptin, our analysis covers drugs representing the vast majority of the DPP4 market (97–98%) and avoids the impact of confounding factors associated with drugs taken by relatively few patients (2–3%) prior to May, 2015.
TABLE 1.
Summary of FAERS search analysis
| Search terms | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Drug | Reports | Events | Ketoacidosis | Acidosis (including metabolic and lactic) |
Cases with quantifiable data (present analysis) |
FDA confirmed cases9 (March 2013-May 2015) |
| SGLT2 inhibitors | ||||||
|
| ||||||
| Cana | 5371 | 11 028 | 137 | 50 | 39 | 48 |
|
| ||||||
| Dapa | 2061 | 4865 | 49 | 16 | 11 | 21 |
|
| ||||||
| Empa | 404 | 739 | 6 | 1 | 1 | 4 |
|
| ||||||
| Total | 7836 | 16 632 | 192 | 67 | 51 | 73 |
| All search terms: 259 | ||||||
|
| ||||||
| DPP4 inhibitors | ||||||
|
| ||||||
| Saxa | 4292 | 9012 | 7 | 21 | ND | NA |
|
| ||||||
| Sita | 26166 | 80 787 | 64 | 385 | ND | NA |
|
| ||||||
| Total | 30458 | 89 799 | 71 | 406 | NA | NA |
| All search terms: 477 | ||||||
Although it would be most informative to know the precise number of patients experiencing ketoacidosis events, the FAERS database is limited to case reports (hereby termed “reports”) of ketoacidosis events. The MedDRA® defines the relationship between an event and a (case) report as, “the total number of events may be greater than the number of cases because a case may describe more than one event [e.g. nausea and rash]”. It is not known what percentage of events are correctly diagnosed by physicians and subsequently reported to FAERS. Our analysis implicitly assumes that similar percentages of ketoacidosis events would be reported to FAERS—independent of which drug it was associated with.
We developed a scoring system to characterize the quality of data available to support the evidence of ketoacidosis: Level 1: ketoacidosis with ketone body levels ≥5 mEq/L; Level 2: acidemia (pH <7.3 and/or anion gap >10 (or base excess > −2) and/or serum bicarbonate <18 mmol/L); Level 3: ketonemia only; Level 4: ketonuria only. In Levels 3 and 4, data on parameters such as pH, bicarbonate, or anion gap were not included in the report. The absence of data does not necessarily imply that the parameters were within normal limits.
To estimate the risk associated with a specific drug, it is necessary to relate the number of cases to the number of patient-years of exposure to drug. However, data on the total number of patients treated with specific drugs are not freely available in the public domain. Thus, we employed data on drug sales as a surrogate index of patient exposure. We hypothesize that total sales of drugs are roughly proportional to the number of treated patients inasmuch as the list prices are similar for all drugs included in this analysis. We acknowledge that there can be substantial variation in the actual prices paid by individual patients for the same drug. However, for the purpose of our analysis, we implicitly assume that the patient-to-patient variation may be similar in magnitude among the various SGLT2 inhibitors and DPP4 inhibitors. We obtained data on the value of sales from companies’ financial statements from each company’s website (see Online Supplement 2).17–21 We obtained data on drug prices from the internet (www.goodrx.com).
3 | RESULTS
The FAERS database contained almost 8,000 individual reports on adverse events related to the 3 SGLT2 inhibitors currently on the US market (canagliflozin, dapagliflozin and empagliflozin), and ~30 000 reports for the 2 most commonly prescribed DPP4 inhibitors (saxagliptin and sitagliptin) from the time of drug approval until May, 2015. Applying various search terms for acidosis, we identified 259 (95% CI: 227–29) and 477 (95% CI: 433–521) reports for SGLT2 and DPP4 inhibitors, respectively (Table 1). Similarly, applying the more specific search term “ketoacidosis”, we identified 192 (95% CI: 164–200) and 71 (95% CI: 54–88) reports for SGLT2 inhibitors and DPP4 inhibitors, respectively. We reviewed all 192 SGLT2 inhibitor-associated ketoacidosis reports and selected a subset of 51 reports based on availability of specific laboratory results supporting a diagnosis of ketoacidosis (Table 2). In December, 2015, the FDA confirmed 73 reports of ketoacidosis in SGLT2 inhibitor-treated patients requiring hospitalization or emergency department visits. The difference between the FDA report11 and our analysis may be explained by differences in selection criteria.
TABLE 2.
Reports of diabetic ketoacidosis from FDA date of approval
Of the 51 cases, T1D accounted for 20 (39%) and T2D for 25 (49%); 6 cases (12%) did not specify the diabetes type. All 20 patients identified with the diagnosis of T1D received insulin, 3 of them in combination with metformin (presumably prescribed off label). Seven of the 25 patients identified with the diagnosis of T2D received insulin. Seventeen patients with T2D took metformin (4 in combination with insulin). Of the 51 reports, 41 provided sufficient glucose information to document that 29 (71%) either fit criteria for euglycemic ketoacidosis or explicitly stated that the patient had “euglycemic DKA”.
Ten reports (20%) provided the highest grade of evidence (Level 1) —ie, circulating ketone body levels >5 mmol/L. The majority (59%) provided Level 3 evidence—specifically, 1 or more of the following: pH < 7.3, anion gap >10 mEq/L, base excess <−2, and/or serum bicarbonate <18 mmol/L. Ten reports (20%) included only information on ketonuria (Level 4 evidence).
In addition to the laboratory results, some case reports included a detailed clinical narrative, while others simply stated the diagnosis. The reports included multiple scenarios describing dehydration, flu-like symptoms, gastroenteritis, recent surgery, low carbohydrate diet, insulin dose reduction, etc. as potential factors related to the development of ketoacidosis (see Online Supplement 3). Fourteen cases (27%) did not identify any precipitant for ketoacidosis other than SGLT2 inhibitor therapy.
3.1 | Comparative analysis
We estimate that cumulative sales for SGLT2 inhibitors (2013-1H/2015) were ~$1.5 billion vs ~$38 billion for the 2 DPP4 inhibitors (2006-1H/2015) (Table 3). Assuming an annual price of ~$4300 for both SGLT2 and DPP4 inhibitors, we estimate that sales for SGLT2 inhibitors correspond to ~0.35 million patient-years vs ~9 million patient-years for DPP4 inhibitors. Total sales of DPP4 inhibitors (2006–2015) were approximately 25-fold greater than total sales of SGLT2 inhibitors (2013–2015). Using total drug sales as a surrogate index of patient exposure, we estimate the incidence of reports of acidosis is ~14-fold higher with SGLT2 inhibitors than for DPP4 inhibitors. T1D patients represented a significant percentage of SGLT2 inhibitor-treated patients in FAERS—ie, 39% of acidosis reports (see above). Because it is established that T1D is associated with a substantially greater risk of ketoacidosis, we conducted an analysis focusing exclusively on the 49% of acidosis reports explicitly stated to occur in T2D patients treated with SGLT2 inhibitors. By contrast, we assumed that all of the reported cases of acidosis in DPP4 inhibitor treated patients occurred in the context of T2D. Based on these assumptions, the corrected analysis provides a lower estimate for the SGLT2 inhibitor-associated risk of acidosis inT2D patients—ie, a 7-fold (rather than a 14-fold) increase in risk.
TABLE 3.
Risk estimate of acidosis and ketoacidosis in SGLT2 inhibitor and DPP4 inhibitor treated patients related to drug sales
| SGLT2 inhibitors | Risk analysis | DPP4 inhibitors (sitagliptin and saxagliptin) |
|---|---|---|
| 259 (192) | Reports of acidosis (ketoacidosis) | 477(71) |
| $1.5 billion | Total sales (approval— May, 2015) | $38 billion |
| 173 | Risk (reports per billion dollars of sales) | 13 |
| 345,000 | Estimated patient years (extrapolated from sales) | 8 740 000 |
| 0.75 (0.55) | Reports per est. thousand patient years | 0.055 (0.008) |
It is also noteworthy that 74% of the reports of SGTL2 inhibitor-associated reports of acidosis were designated as “ketoacidosis” compared with only 15% of DPP4 inhibitor-associated reports. This raises the possibility that a significant fraction of DPP4 inhibitor-associated cases of acidosis may represent cases of lactic acidosis rather than ketoacidosis.
4 | DISCUSSION
The present analysis confirms the FDA’s conclusion that SGLT2 inhibitors increase the risk of ketoacidosis, and extends the previously available information in 3 ways: (1) by providing comparator data derived from analyses of reports of adverse events in patients treated with another class of antidiabetic drugs (DPP4 inhibitors), (2) by providing available evidence in a transparent manner, and (3) by relating the number of reports to estimates of patient-years of exposure to specific drugs.
Ketoacidosis continues to be an issue for adults with T1D. Estimates from the T1D Exchange Clinic Registry22 include 1 or more DKA events within 12 months reported by 4.8% of 6796 participants, which correlated with their HbA1c (going as low as 1.6% for HbA1c < 6.5% to up to 21% for HbA1c > 10%). Risk factors include younger age, poorer glycemic control, lower socioeconomic status, female gender and race, among others. Previous studies have shown that the placebo-subtracted incidence of serious diabetic ketoacidosis in SGLT2 inhibitor-treated T1D patients ranged from 4.3 to 6.0% in a randomized control trial with canagliflozin (100–300 mg/day) over 18 weeks.8 Furthermore, in addition to increasing the risk of serious ketoacidosis, SGLT2 inhibitors also increased the risk of serious hypoglycemia inT1D patients in that study. Of the 51 reports in our detailed analysis, 39% related to patients withT1D whereas 12% did not specify the diabetes type. If these cases (ie, either T1D or unspecified type of diabetes) are excluded from the analysis, then the risk in T2D patients would be 51% lower—ie, ~7-fold increased risk based on all cases of acidosis in T2D (instead of 14-fold). These estimates approximately double the previously reported ~3-fold increased risk.9
Widespread public awareness of the potential risk for ketoacidosis was generated by the first FDA announcement in May 2015. By ending our analysis in May 2015 and by analysing the data according to both search terms “acidosis” and “ketoacidosis”, we attempted to minimize bias that could have been introduced—eg, by an increased number of reports generated by health care providers who were sensitized to the issue or, by contrast, by a decreased number of reports due to a perceived lack of novelty. In addition, there was an approximate 2-year overlap in the reporting period for both drug classes. During this time, published data on the risk had not become available; thus, clinicians probably did not suspect that these 2 classes of drugs would have different risks for treatment-emergent ketoacidosis. Therefore, it is reasonable to assume that physicians would be equally likely to report cases of ketoacidosis—regardless of whether a patient was being treated with a DPP4 inhibitor or an SGLT2 inhibitor.
In order to estimate reporting rates for acidosis, it is necessary to relate the number of reports to the number of patient-years of exposure. Unfortunately, companies do not include data on the numbers of patients who take their drugs. However, government regulations require companies to disclose data on drug sales if the sales are large enough to be of significance to the companies’ financial health. We have used total drug sales as an index to estimate patient years of exposure. During the timeframe under analysis, sitagliptin and canagliflozin account for ~89% and ~93% of total sales for the DPP4 inhibitor and SGLT2 inhibitor classes, respectively, and sales of these 2 drugs are the major determinants of total sales for the 2 classes of drugs. Both drugs have similar list prices (~$4000 per year). For most patients, the actual selling price would be discounted relative to the list price. Our analysis implicitly assumes that the average discounted price would be similar for drugs in both classes. Although we recognize that there may be some degree of variation in the size of discounts among the various drugs in these 2 classes, it seems unlikely that the variation is sufficient to account for the estimated differences in incidences of reported cases of acidosis. For example, if the average selling price for sitagliptin were discounted by 50%, then one would need to assume that the average selling price for canagliflozin would have been discounted by 93% to account for the 7-fold difference in the incidence estimates. Such a large difference in price discount seems unlikely for 2 first-in-class diabetes drugs such as sitagliptin vs canagliflozin.
Erondu et al suggested that 50% of cases of diabetic ketoacidosis occurred in patients with anti-GAD65 autoantibodies, who are presumed to have adult onset autoimmune mediated diabetes rather than non-autoimmune T2D.9 Likewise, others23 have stated the risk for ketoacidosis with SGLT2 inhibitors “may be increased in long-standing T2D patients with marked β-cell insufficiency or in latent autoimmune diabetes in adults with rapid evolution toward T1D”. From a purely academic perspective, this is an important finding, which has potential to give insight into the mechanisms of the pathogenesis of SGTL2 inhibitor-induced diabetic ketoacidosis. However, in order to translate this into a clinically relevant risk management strategy, it would be necessary to routinely measure anti-GAD65 antibodies or c-peptide levels prior to prescribing SGLT2 inhibitors. This analysis estimates that SGLT2 inhibitors are associated with an ~7-fold increase in risk of acidosis in type 2 diabetic patients, which is approximately double the 2-fold to 4-fold increase in the numerical imbalance reported for canagliflozin9 and empagliflozin.1 It is not entirely surprising that the risk might be somewhat higher in real world practice than observed in formal clinical studies in which patients are highly screened and have especially good access to skilled clinical care. Moreover, we acknowledge that our use of a surrogate index for patient exposure does not provide the same degree of certainty that is available in a randomized controlled trial. In addition, it is possible that in real-world practice, there may be differences between patients who receive DPP4 inhibitors vs SGLT2 inhibitors. Nevertheless, we are reassured that our estimates of the increased risk are in the same “ball park” as the published real world experience with canagliflozin and empagliflozin.
Plausible biological mechanisms for the increased risk of ketoacidosis include increased glucagon levels,24 possibly mediated by a direct effect on pancreatic alpha-cells.25 Given the role of glucagon in regulating hepatic ketogenesis, SGLT2 inhibitor-induced glucagon secretion would be predicted to increase serum ketone body concentrations. Other contributing factors may include the net negative glucose balance, leading to a shift away from glucose oxidation, thereby promoting fatty acid oxidation, and ketogenesis which increases circulating ketone bodies.26–28 Furthermore, it is now well established that SGLT2 inhibitors elevate levels of circulating ketone bodies in patients with type 2 diabetes (T2D).26,28,29 Moreover, in recent studies with luseogliflozin, it has been demonstrated that a low carbohydrate diet exacerbates the SGLT2 inhibitor-induced increase in circulating ketone body levels.30 Although the degree of ketonemia did not reach levels sufficient to cause major disturbances in acid-base balance, it seems plausible that this degree of druginduced ketosis might predispose patients to develop overt ketoacidosis—especially in the presence of an independent precipitating factor such as infection or the stress associated with surgery.
Additional factors have potential to introduce confounders with respect to our estimates of the reporting rate expressed as a function of total sales for a drug. For example, it is theoretically possible that susceptible patients might experience drug-induced ketoacidosis early during the course of receiving SGLT2 inhibitor therapy. If an episode of ketoacidosis were to lead to discontinuation of the drug, this has potential to provide powerful selection such that patients receiving long-term therapy would tend to have low susceptibility to develop ketoacidosis. However, as reported by Fadini et al,15 reports of ketoacidosis can occur after any duration of SGLT2 inhibitor use. This observation suggests that any differences between short-term vs long-term therapy is unlikely to introduce significant bias in occurrence of ketoacidosis events or ascertainment of reports. Additionally, with the increased public awareness of SGLT2 inhibitor-induced ketoacidosis, it is plausible that physicians were more likely to test for ketonuria or ketonemia in patients on SGLT2 inhibitor therapy. We have relied on the reports for diagnosis of T1D vs T2D. Furthermore, we have not attempted to distinguish latent autoimmune diabetes of adults (LADA) from more typical T2D. Although it is possible that some of the patients identified as T2D might have had autoimmune disease, nevertheless, we are following the diagnosis they received in routine clinical practice. Conversely, 3 of the 20 patients diagnosed as T1D were receiving metformin. We have implicitly assumed that these 3 patients were indeed T1D patients receiving metformin off-label just as they were receiving SGLT2 inhibitors off-label. Nevertheless, if they were misclassified and should have been diagnosed as T2D, this would have the effect of increasing the number of ketoacidosis reports in T2D patients.
FDA has required labelling for all members of the SGLT2 inhibitor to indicate a risk for ketoacidosis. Indeed, the data in the FAERS database are consistent with the inference that it is a class effect. This contrasts with respect to certain other adverse effects (ie, bone loss, fracture, and amputations) where the labelling is currently restricted to canagliflozin. While all 3 approved SGLT2 inhibitors share similar C-glucoside chemical structures, it is possible that there could be some degree of differentiation within the class. For example, in a head-to-head trial, approved doses of canagliflozin demonstrate greater efficacy than dapagliflozin.31 It is possible that the observed difference is explained by the fact that the highest approved dose of canagliflozin is at the upper plateau of the dose-response curve whereas the highest approved dose of dapagliflozin achieves only sub-maximal efficacy. Further, it is possible that the approved SGLT2 inhibitors might be differentiated with respect to selectivity for other SGLT-family members (eg, SGLT1, SGLT3, and SGLT4).
It can be challenging for Regulatory Agencies, including the FDA, to detect safety signals in pre-approval clinical trials, which typically include comparatively healthy patients treated for relatively short times (6–12 months). In addition, phase 3 studies are generally too small to provide compelling statistical evidence to detect an uncommon safety signal (eg, with an incidence of ≤1:1000), particularly because safety endpoints are rarely pre-specified. Nevertheless, the FAERS database provides an opportunity to evaluate post-marketing “real-world” cases. While FAERS faces its own challenges, including the well-known limitation in the ability to define a “real incidence”, it is an important tool to identify safety risks. In light of public interest in the benefit:risk profiles for approved drug—especially drugs as widely used as SGLT2 inhibitors—it is useful to provide insight into the data that support FDA’s safety evaluation.32
The Institute of Medicine report on drug safety33 identifies challenges in all aspects of drug review and highlights that the FDA is limited in its ability to take “systematic approach to identify possible pre-marketing drug-safety problems and translate them into high-quality post-marketing studies,” but depends on requests to sponsors to conduct expensive post-approval trials.34 The tools (eg, FAERS) to detect post-marketing safety signals are dependent on spontaneous reporting, which does not necessarily provide data of the highest quality. Troglitazone, the first thiazolidinedione approved in the United States proved to be hepatotoxic post-approval and was subsequently withdrawn. Likewise, thiazolidinedione-related fractures required time to develop due to the inherent nature of bone physiology.35,36 More recently, considerable public concern focused on a debate as to whether or not DPP4 inhibitors cause pancreatitis, which was complicated to tease out in the post-marketing system.37 The FDA is to be commended for its leadership in identifying safety concerns for this new class of drugs, and also for communicating concerns to physicians and patients. Nevertheless, once FDA has communicated its conclusions, this causes stakeholders to ask questions about the nature and the strength of the evidence supporting those conclusions. Although the FAERS database contains highly relevant data, that database is not freely accessible online. We are grateful that FDA has provided us with the reports, thereby enabling us to analyse the data and make our analysis available in the peer-reviewed literature.
Supplementary Material
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). We would like to thank Marc Reitman for helpful scientific discussions. We would also like to thank Sarah Lock (NIDDK, summer student) for her assistance in preparing and extracting data from the FAERS reports. JEB, SIT, SHT, and KIR are responsible for the design of the data analysis. All authors (JEB, SIT, SHT, and KIR) had full access to all of the data provided by FDA and take responsibility for the integrity and the accuracy of the data analysis. SIT was previously employed by Bristol-Myers Squibb Company and has served as a paid consultant or advisor for Aegerion Pharmaceuticals and Ionis Pharmaceuticals.
Footnotes
JEB, SHT, and KIR declare no competing interests.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 4.AstraZeneca. Multicenter trial to evaluate the effect of Dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI58) 2017 Jan; Available from: https://clinicaltrials.gov/ct2/show/NCT01730534?term=dapagliflozin+outcome&rank=6.
- 5.Invokana prescribing information. Available from: www.invokanahcp.com/prescribing-information.pdf.
- 6.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with Canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of Canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258–2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 9.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the Canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015 doi: 10.2337/dc15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(FDA), F.a.D.A, FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015 [Google Scholar]
- 11.(FDA), F.a.D.A, FDA drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015 Dec 4; [Google Scholar]
- 12.Endocrinologists, A.A.O, AACE/ACE scientific and clinical review: association of SGLT2 inhibitors and DKA. 2015 [Google Scholar]
- 13.Jensen ML, Persson F, Andersen GS, et al. Incidence of ketoacidosis in the Danish type 2 diabetes population before and after introduction of sodium-glucose cotransporter 2 inhibitors—a nationwide, retrospective cohort study, 1995–2014. Diabetes Care. 2017;40(5):e57–e58. doi: 10.2337/dc16-2793. [DOI] [PubMed] [Google Scholar]
- 14.Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376(23):2300–2302. doi: 10.1056/NEJMc1701990. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA adverse event reporting system. Diabetologia. 2017 doi: 10.1007/s00125-017-4301-8. [DOI] [PubMed] [Google Scholar]
- 16.FDA. [December 4, 2015];Drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2016 Jun 9; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm.
- 17.Boehringer-Ingelheim.com. Financial reports. 2015 Jul 30; Available from: https://www.boehringer-ingelheim.com/corporate_profile/annual_report.html.
- 18.Janssenbelgium.be. Annual report. 2015 Jul 30; Available from: http://www.janssenbelgium.be/en/media-center/documents/annual-report-jj-2013.
- 19.Lilly E. Annual reports and financials. 2015 Jul 30; Available from: https://investor.lilly.com/annuals.cfm.
- 20.AstraZeneca.com. Financial reports. 2015 Jul 30; Available from: http://www.astrazeneca.com/Investors/financial-information/Financial-results.
- 21.Information, T.I. Financial information. 2015 Jul 30; Available from: https://www.takeda.com/investor-information/annual/
- 22.Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411–3419. doi: 10.1210/jc.2013-1589. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638–1642. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21(5):512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 27.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 28.Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65. doi: 10.1186/1475-2840-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniele G, Xiong J, Solis-Herrera C, et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care. 2016;39(11):2036–2041. doi: 10.2337/dc15-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabe D, Iwasaki M, Kuwata H, et al. SGLT2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open-label, 3-arm parallel comparative exploratory study. Diabetes Obes Metab. 2017;19(5):739–743. doi: 10.1111/dom.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188–197. doi: 10.1111/dom.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhatariya K. Comment on Erondu et al. Diabetic ketoacidosis and related events in the Canagliflozin type 2 diabetes clinical program. Diabetes care 2015;38:1680–1686. Diabetes Care. 2016;39(1):e18. doi: 10.2337/dc15-1956. [DOI] [PubMed] [Google Scholar]
- 33.Challenges for the FDA: The Future of Drug Safety, Workshop Summary. Washington DC: National Academy of Sciences; 2007. [PubMed] [Google Scholar]
- 34.Psaty BM, Burke SP. Protecting the health of the public–Institute of Medicine recommendations on drug safety. N Engl J Med. 2006;355(17):1753–1755. doi: 10.1056/NEJMp068228. [DOI] [PubMed] [Google Scholar]
- 35.Bazelier MT, Vestergaard P, Gallagher AM, et al. Risk of fracture with thiazolidinediones: disease or drugs? Calcif Tissue Int. 2012;90(6):450–457. doi: 10.1007/s00223-012-9591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolman KG. The safety of thiazolidinediones. Expert Opin Drug Saf. 2011;10(3):419–428. doi: 10.1517/14740338.2011.534982. [DOI] [PubMed] [Google Scholar]
- 37.Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther Adv Drug Saf. 2014;5(3):138–146. doi: 10.1177/2042098614523031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.