Abstract
The amount of bone gained during childhood and adolescence impacts greatly on lifetime skeletal health. The purpose of this review is to summarize current evidence of the effects of gymnastics activities on bone mineral accrual during growth and to describe possible factors that influence bone mineral gains. The PubMed and SportDiscus databases were searched, and a total of 24 articles met the selection criteria and were included in this review. Artistic and rhythmic gymnasts presented higher bone mineral density and content values compared to untrained controls, despite possible negative effects associated with hormonal levels, dietary restrictions and body fat. The results suggest that gymnasts had similar bone turnover values compared to untrained controls. High-intensity mechanical loading of gymnastics activity appears to increase bone development and counterbalance negative effects, such as later pubertal development, lower body fat mass and lower hormone levels. In conclusion, gymnasts present higher bone mineral values in comparison with untrained controls. The osteogenic effect of gymnastics athletic activity has a positive influence on bone mineral accrual and overcomes the possible negative influence of high athletic activity that may cause negative energy balance and low body fat mass which are associated with lower bone accrual.
Key points.
Children and adolescent gymnasts present higher bone mineral density and content values compared to untrained controls, despite a variety of possible negative factors.
Gymnastics activity with high-impact mechanical loading appears to be especially osteogenic to achieve maximum possible peak bone accrual during growth and maturation.
Skeletal benefits of gymnastics activity in childhood are maintained for several years after retirement from gymnastics trainings in young adulthood.
Key words: Gymnasts, training, growth and maturation, bone mineral accrual, bone turnover
Introduction
The amount of bone gained during childhood impacts greatly on lifetime skeletal health (Gruodyte-Raciene et al., 2013). Bone is a metabolically active tissue with continuous remodeling occurring throughout the lifespan (Jürimäe, 2010). Bone mineral accrual increases substantially during the growing years peaking in velocity of accrual (peak bone mineral content velocity [PBMCV]) seven months after the attainment of peak height velocity (PHV) (Bailey, 1999). Bone mass then plateaus between the end of the second decade of life and the middle of the third; termed peak bone mass (PBM) (Bailey, 1999). Individuals who achieve a higher PBMCV during adolescence and a subsequently higher PBM have decreased fracture risk in later life (Weaver et al., 2016; Xu et al., 2016). Maximal increases in bone mineral accrual occur over a relatively brief period in the years surrounding PHV (Baxter-Jones et al., 2011; Jackowski et al., 2011a). Specifically, Baxter-Jones et al. (2011) showed that up to 40% of peak bone was accrued in a five year window surrounding attainment of PHV. Accordingly, puberty is an opportune time for bone strengthening (Jackowski et al., 2011a; Vaitkeviciute et al., 2014), and research should focus on strategies for maximizing peak bone mineral accrual during this growth period in order to lay the foundation for better adult bone health.
The accumulation of bone mineral during pubertal growth is influenced by a number of factors, including but not limited to: timing of pubertal maturation (Jackowski et al. 2011a), body composition (Ivuskans et al., 2013), nutritional status (Maimoun et al., 2014), endocrine function (Pomerants et al., 2007), habitual physical activity (Vaitkeviciute et al., 2016) and athletic training (Võsoberg et al., 2016). Therefore, some of these factors may influence bone mineral accrual negatively and some positively. The decrease in overall physical activity and increase in daily sedentary time has been shown to negatively influence bone mineral accrual in boys during puberty (Ivuskans et al., 2015; Vaitkeviciute et al., 2014), while mechanical loading of athletic training is a positive factor for skeletal strength and bone development during the same time period (Gruodyte et al., 2010a; Parm et al., 2011a; 2011b). In addition to the independent effect of mechanical loading on bone mass and density, increased mechanical loading as a result of athletic training may also influence structural changes in bone to increase bone strength in response to specific loading conditions (Weaver et al., 2016). Therefore, the effects of sport on bone health in children vary in relation to training modality, ranging from non-weight bearing (swimming) and low-impact activities (skiing) to high-impact activities (gymnastics) (Gruodyte et al., 2009; 2010b). Accordingly, regular high-impact weight-bearing athletic activity during pubertal growth plays an important role in maximizing bone mineral gain and may reduce the risk of osteoporosis in later life (Baxter-Jones et al., 2008; Erlandson et al., 2012a; 2012b). It appears that gymnastics training is especially osteogenic for bone development in children (Gruodyte-Racience et al., 2013), adolescents (Gruodyte et al., 2009) and adults (Sööt et al., 2005), probably due to the high-volume, high-impact training and involvement at a relatively early age during growth (Tournis et al., 2010). However, intense athletic activity in growing and maturing gymnasts imposes several constraints such as training stress and maintenance of a relatively low fat mass (FM) in order to maximize gymnastics performance (Maimoun et al., 2014). Pubertal gymnasts are at risk of inadequate dietary intake, which in turn may have several consequences for endocrine function (Jürimäe, 2014; Malina et al., 2013). Accordingly, a serious question is raised about the positive effects of regular gymnastics activities on overall health and especially normal bone mineral accrual during the growing years in age related elite, sub-elite and recreational gymnasts. It is not clear how prolonged high gymnastics activity during growth and maturation affects bone health later in adulthood.
To date, bone health in gymnasts has been measured by different imaging techniques. The assessment of bone health includes the two-dimensional measurement of areal bone mineral density (aBMD) and bone mineral content (BMC) measured by dual-energy X-ray absorptiometry (DXA) and/or the three-dimensional measurement of volumetric BMD (vBMD) and bone strength indices measured by peripheral quantitative computed tomography (pQCT) (Xu et al., 2016). DXA scans have also been used to assess the structural geometry of the proximal femur using the hip structural analysis program (Beck et al., 1990; Jackowski et al., 2009; 2011b). However, these imaging measurements provide only a static representation of bone tissue. Accordingly, in addition to static measure of bone tissue, it is also suggested that measures of a more dynamic nature are taken to better describe bone development during growth and maturation, which include blood biochemical markers of bone formation and resorption (Jürimäe, 2010). One of the limitations using bone formation and resorption markers is that these markers represent an average turnover from all skeletal sites of the body and consequently are not site specific (Jürimäe, 2010). However, it can be argued that to experience an increase in bone mineral values, elevations in bone formation markers would be necessary to overcome the increase in bone resorption markers (Jürimäe, 2010). In addition, the knowledge of pubertal growth is necessary to correctly interpret the values of bone turnover, as the highest levels of bone turnover markers are observed at early puberty (Vaitkeviciute et al., 2016). It seems necessary to analyze the effect of childhood gymnastics athletic activity on bone mineral acquisition using both static and dynamic measures of bone development, as 60% of osteoporosis cases in adulthood are related to low bone mineral acquisition during childhood (Saggese et al., 2001).
To our knowledge, there is no overview of the specific effects of gymnastics activities on bone mineral accrual during growth and maturation in order to prevent possible osteoporosis in later adulthood. Therefore, we performed the present overview in order to assemble current evidence on this topic. Specifically, the aims of the current systematic review were to determine the differences in aBMD and BMC accrual between gymnasts and controls during the growing years and to describe different factors that could influence bone accrual.
Methods
Data sources and search strategy
This review followed the systematic review methodology proposed in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009). Studies were identified by searching within the electronic databases (Gomez-Bruton et al., 2013). The identification of studies was performed by searching within PubMed and SportDiscus (Gomez-Bruton et al., 2016). The search was conducted up to September 2017. The first search was performed using the thesaurus provided by both databases, while the second search was performed with the following combination of terms: gymnasts and bone density (Gomez-Bruton et al., 2013; 2016). Two reviewers independently examined both databases to obtain the potential publications. Relevant articles were obtained in full, and assessed against inclusion and exclusion criteria described below. Inter-reviewer disagreements were resolved by consensus. Arbitration by a third reviewer was used for unresolved disagreements (Gomez-Bruton et al., 2013; 2016; Sioen et al., 2016).
Inclusion criteria
The following inclusion criteria were used (Gomez-Bruton et al., 2013; 2016): 1) types of study designs: cross-sectional and longitudinal studies investigating the effects of gymnastics training programs on bone mass; 2) types of participants: children and adolescents; and 3) types of outcome measured: aBMD, BMC and bone area (BA) of the whole body (WB), lumbar spine (LS), femoral neck (FN) and forearm (FA) measured by DXA, and bone architecture of the tibia and radius measured by pQCT.
Exclusion criteria
The following exclusion criteria were used (Gomez-Bruton et al., 2013; 2016): 1) studies without a control group that would permit comparison, 2) studies that measure BMD/aBMD but do not give specific WB, LS, FN or FA values, 3) studies that focus exclusively on bone metabolism markers without measuring bone with an imaging technique, 4) congress abstracts, dissertations and other similar unpublished data, 5) studies in languages other than English.
Data extraction
One author independently extracted bone density data from the included studies. The DXA-derived bone density data included in this review were obtained from the four frequently reported DXA scans: WB, LS, FN and FA. LS measures were taken from L1 to L4 or L2 to L4. Bone density values were generally extracted from the tables reported by the included papers in the systematic review. Estimates of volumetric bone density calculated by DXA (bone mineral apparent density), reported by some studies were not extracted. All included studies presented raw data.
Quality assessment
Quality assessment of all studies included in this systematic review was done using the same quality assessment tool as Olmedillas et al. (2012), which grades articles on a scale of 7 points. This quality assessment tool has also been used in previous systematic reviews (Gomez-Bruton et al., 2013; 2016).
Search summary
The initial search strategy identified 790 potentially relevant articles. Following the review of article titles and abstracts, and also excluding duplicate articles, where the same study results were described in more than one research article, the total number of articles was reduced to 75 potentially relevant papers for the inclusion to the review. Of these articles, 24 articles met the selection criteria and were included in this review (Figure 1).
Figure 1.
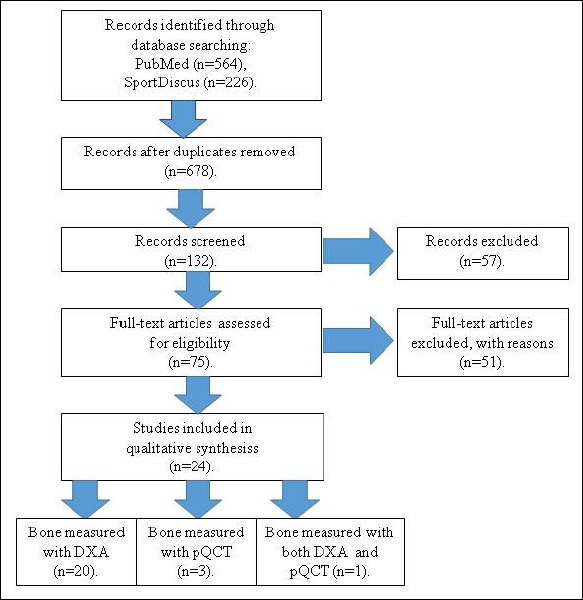
PRISMA flow diagram.
Results
Characteristics of included studies
A total of 1434 participants (731 gymnasts and 703 sedentary controls) from the 24 studies (Tables 1 and 2) were included in this systematic review. Among gymnasts, 264 were rhythmic gymnasts (RG) and 467 were artistic gymnasts (AG). Majority of participants were female subjects as only 10 male AG and 10 male untrained controls (UC) were included into the review. The quality assessment of studies revealed that most studies (21 studies) were graded as 4/7 (Burt et al., 2012; Courteix et al., 1998; Dowthwaite et al., 2006; 2011; Dyson et al., 1997; Erlandson et al., 2012a; Greene et al., 2012; Gruodyte et al., 2010a; Jürimäe et al., 2016; Lehtonen-Veromaa et al., 2000a; Maimoun et al., 2011; 2013a; Munoz et al., 2004; Nickols-Richardson et al., 1999; Nurmi-Lawton et al., 2004; Parm et al., 2011b; Pikkarinen et al., 2009; Tournis et al., 2010; Vicente-Rodriguez et al., 2007; Võsoberg et al., 2016; Zanker et al., 2003), with three studies being graded as 5/7 (Cassell et al., 1996; Courteix et al., 2007; Maimoun et al., 2013b). These moderate quality assessment scores in studies that were included to our systematic review were in line with the results obtained by Gomez-Bruton et al. (2016), who found similar moderate scores in the studies that they included in their systematic review of the effect of swimming on BMD during childhood and adolescence.
Table 1.
Descriptive characteristics of included studies with rhythmic gymnasts.
| Study | Participants | Training | Data source | Measured areas | Outcome | QA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Sex | Age (yrs) | Tanner stage | History (yrs) | Volume (hours/week) | |||||
| Parm et al. (2011b) | RG (46) UC (43) |
F F |
8.0±0.6 8.2±0.6 |
I I |
1-3 | 6-12 | DXA | Whole body, lumbar spine, femoral neck. | RG had higher WB aBMD, LS aBMD and FN aBMD compared to UC | 4/7 |
| Jürimäe et al. (2016*) | RG (32) UC (32) |
F F |
10.0±0.6 10.1±0.5 |
32/0/0/0/0 12/14/6/0/0 |
≥4 | 10-12 | DXA | Whole body, lumbar spine, femoral neck. | RG had higher WB aBMD, LS aBMD and FN aBMD compared to UC | 4/7 |
| Vicente-Rodriguez et al. (2007) |
RG (13) UC (13) |
F F |
10.4±0.7 9.9±0.7 |
3/10/0/0/0 2/11/0/0/0 |
3.3±1.2 | 15 | DXA | Whole body, lumbar spine, femoral neck, forearm. | RG had higher FN aBMD and FN BMC compared to UC | 4/7 |
| Võsoberg et al. ((2016) | RG (35) UC (33) |
F F |
10.9±0.6 11.2±0.5 |
8/23/4/0/0 4/15/10/4/0 |
≥5 | 6-14 | DXA | Whole body, lumbar spine, femoral neck. | RG had higher WB aBMD, LS aBMD, FN aBMD and WB BMC compared to UC | 4/7 |
| Tournis et al. (2010*) | RG (26) UC (23) |
F F |
11.3±0.2 10.9±0.1 |
10/13/3/0/0 7/12/4/0/0 |
4.3±0.3 | ≥24 | pQCT | Distal tibia. | RG had higher cortical and trabecular BMC, and trabecular vBMD compared to UC | 4/7 |
| Maimoun et al. (2013a*) | RG (20) UC (20) |
F F |
13.8±2.2 13.7±2.0 |
3/7/3/0/7 2/3/3/1/11 |
≥5 | 21.4±4.4 | DXA | Whole body, lumbar spine, femoral region, radius. | RG had higher FN aBMD compared to UC | 4/7 |
| Courteix et al. (2007*) | RG (36) UC (20) |
F F |
13.4±1.8 12.5±1.7 |
I-V | N/A | 18.1±3.3 | DXA | Whole body, lumbar spine | RG had higher WB aBMD and LS aBMD compared to UC | 5/7 |
| Maimoun et al. (2013b*) | RG (24) UC (24) |
F F |
13.9±1.7 14.4±1.8 |
4/4/6/4/6 1/1/2/2/18 |
6.8±1.3 | 23.0±2.7 | DXA | Whole body, lumbar spine, femoral neck, forearm | RG had higher WB aBMD and FN aBMD compared to UC | 5/7 |
| Gruodyte et al. (2010a) | RG (23) UC (33) |
F F |
14.3±1.0 14.2±1.1 |
0/1/7/11/4 0/1/5/23/4 |
6.5±1.8 | 9.6±4.9 | DXA | Lumbar spine, femoral neck | RG had higher LS aBMD and FN aBMD compared to UC | 4/7 |
| Munoz et al. (2004*) | RG (9) UC (14) |
F F |
16.2±2.0 16.9±1.0 |
V V |
≥5 | ≥20 | DXA | Lumbar spine, femoral neck, forearm | RG had higher FN aBMD and lower FA aBMD compared to UC | 4/7 |
Tanner stage: when presented by Roman numbers, it represents what Tanner stages participants were, without specifying the number of participants in each Tanner stage; when presented by Arabic numbers, it represents the number of participants in each Tanner stage acoordingly: I/II/III/IV/V.
* These studies have also measured bone turnover values. aBMD areal bone mineral density, BA bone area, BMC bone mineral content, DXA dual-energy X-ray absorptiometry, F female, FN femoral neck, FA forearm, LS lumbar spine, M male, N/A not available, pQCT peripheral quantitative computed tomography, QA quality assessment, RG rhythmic gymnasts, UC untrained controls, vBMD volumetric bone mineral density, WB whole body.
Table 2.
Descriptive characteristics of included studies with artistic gymnasts.
| Study | Participants | Training | Data source |
Measured areas | Outcome | QA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Sex | Age (yrs) |
Tanner stage |
History (yrs) |
Volume (hours/week) |
|||||
| Zanker et al. (2003) |
AG (10) UC (10) |
M M |
8.1±0.2 7.6±0.1 |
I I |
1-2 | 4-6 | DXA | Whole body, lumbar spine. | AG had similar measured bone values with UC. | 4/7 |
| Zanker et al. (2003) |
AG (10) UC (10) |
F F |
8.1±0.1 7.6±0.1 |
I I |
3-4 | 8-10 | DXA | Whole body, lumbar spine. | AG had higher LS aBMD and compared to UC. | 4/7 |
| Cassell et al. (1996) |
AG (14) UC (17) |
F F |
8.8±0.2 8.3±0.2 |
I I |
≥1 | 13.9 | DXA | Whole body. | AG had higher WB aBMD compared to UC. | 5/7 |
| Dyson et al. (1997) | AG (16) UC (16) |
F F |
9.8±0.9 9.9±0.8 |
I I |
3-7 | 16-23 | DXA, pQCT |
Whole body, lumbar spine, femoral region, radius. | AG had higher LS aBMD and FN aBMD; and total, trabecular and cortical distal radius aBMD compared to UC | 4/7 |
| Courteix et al. (1998) | AG (18) UC (13) |
F F |
10.4±1.3 10.7±1.0 |
I I |
≥3 | 10-15 | DXA | Lumbar spine, femur, radius. | AG had higher LS aBMD, FN aBMD and FA aBMD compared to UC. |
4/7 |
| Dowthwaite et al. (2006) | AG (12) UC (10) |
F F |
10.0±1.0 10.4±0.9 |
I I |
≥2 | 10.3±2.4 | DXA | Lumbar spine, femoral neck, forearm. | AG had higher LS aBMD, FN aBMD, FA aBMD, LS BMC, FA BMC and FA BA compared to UC. | 4/7 |
| Nickols-Richardson et al. (1999*) | AG (9) UC (9) |
F F |
11.0±0.3 11.1±0.3 |
I I |
7.1±0.6 | 15.7±1.6 | DXA | Whole body, lumbar spine, femoral region. | AG had higher WB aBMD, LS aBMD, FN aBMD compared to UC. | 4/7 |
| Lehtonen-Veromaa et al. (2000a) | AG (16) UC (14) |
F F |
11.2±0.7 10.9±0.9 |
I I |
≥4 | ≥7.5 | DXA | Lumbar spine, femoral neck, forearm. | AG had similar measured bone values with UC. | 4/7 |
| Burt et al. (2012) |
AG (30) UC (29) |
F F |
8.9 (6-11) 8.6 (6-11) |
26/4/0/0/0 24/5/0/0/0 |
2.6-3.5 | 9.2-12.0 | pQCT | Radius. | AG had higher FA BMC, distal radius BMC, distal radius aBMD and proximal radius BA compared to UC. | 4/7 |
| Vicente-Rodriguez et al. (2007) | AG (13) UC (13) |
F F |
9.7±1.5 9.9±0.7 |
3/10/0/0/0 2/11/0/0/0 |
3.4±2.8 | ≥12 | DXA | Whole body, lumbar spine, femoral region. | AG had higher FA aBMD compared to UC. | 4/7 |
| Dowthwaite et al. (2006) | AG (16) UC (18) |
F F |
11.4±0.9 11.0±0.8 |
II II |
≥2 | 14.7±5.3 | DXA | Lumbar spine, femoral neck, forearm. | AG had higher FN aBMD, FA aBMD, FN BMC, FA BMC and FA BA compared to UC. | 4/7 |
| Erlandson et al. (2012a) | AG (25) UC (22) |
F F |
11.6±1.9 11.9±1.7 |
I-V I-V |
≥2 | ≥15 | DXA | Whole body, lumbar spine, femoral neck. | AG had higher FN aBMD compared to UC. | 4/7 |
| Nurmi-Lawton et al. (2004) | AG (45) UC (52) |
F F |
11.3±2.3 11.3±1.9 |
I-V I-V |
6.5±2.4 | 20.9±4.5 | DXA | Whole body, lumbar spine. | AG had higher WB aBMD, LS aBMD, WB BMC and LS BMC compared to UC. | 4/7 |
| Pikkarainen et al. (2009) | AC (52) UC (44) |
F F |
13.0±1.7 13.0±1.7 |
I-V I-V |
6.4±2.9 | N/A | DXA | Lumbar spine, femoral neck. | AG had higher LS BMC, FN BMC and FN BA compared to UC. | 4/7 |
| Lehtonen-Veromaa et al. (2000a) | AG (50) UC (46) |
F F |
13.3±1.5 13.8±1.3 |
II-V II-V |
≥6 | ≥9 | DXA | Lumbar spine, femoral neck, forearm. | AG had higher LS aBMD, FN aBMD, LS BMC and FN BMC compared to UC. | 4/7 |
| Maimoun et al. (2011*) | AG (23) UC (23) |
F F |
13.4±2.2 13.2±2.2 |
6/1/3/1/12 5/5/3/0/11 |
≥8 | 19.9±4.1 | DXA | Whole body, lumbar spine, femoral region, radius. | AG had higher WB aBMD, LS aBMD, FN aBMD and FA aBMD compared to UC. | 4/7 |
| Maimoun et al. (2013a*) | AG (20) UC (20) |
F F |
13.8±2.0 13.7±2.0 |
4/1/2/1/12 2/3/3/1/11 |
≥5 | 20.3±4.2 | DXA | Whole body, lumbar spine, femoral region, radius. | AG had higher WB aBMD, LS aBMD, FN aBMD and FA aBMD compared to UC. | 4/7 |
| Greene et al. (2012) |
AG (28) UC (28) |
F F |
13.7±1.8 14.3±1.1 |
2.0±0.3a 3.2±0.8a |
N/A | 14.0±5.2 | pQCT | Tibia, radius. | AG had higher cortical and trabecular BMC, cortical and trabecular vBMD at the tibia and radius compared to UC | 4/7 |
| Dowthwaite et al. (2011) | AG (60) UC (54) |
F F |
14.2 13.8 |
21/10/5/19 19/12/4/6/13 |
≥1 | 10.5 | DXA | Lumbar spine region. | AG had higher LS aBMD, LS BMC and LS BA compared to UC. | 4/7 |
Tanner stage: when presented by Roman numbers, it represents what Tanner stages participants were, without specifying the number of participants in each Tanner stage; when presented by Arabic numbers, it represents the number of participants in each Tanner stage acoordingly: I/II/III/IV/V
aTanner stage given as an average value by the authors.
* These studies have also measured bone turnover values. AG artistic gymnasts, aBMD areal bone mineral density, BA bone area, BMC bone mineral content, DXA dual-energy X-ray absorptiometry, F female, FN femoral neck, FA forearm, LS lumbar spine, M male, N/A not available, pQCT peripheral quantitative computed tomography, QA quality assessment, UC untrained controls, vBMD volumetric bone mineral density, WB whole body.
Comparison of bone mineral values between gymnasts and untrained controls
The present cross-sectional comparisons between RG and UC (Table 1), and AG and UC (Table 2) demonstrated that gymnasts had higher measured bone mineral values than age-matched UC. The present review of published articles clearly suggests that systematic gymnastics training has a positive effect on aBMD and BMC values during growth and maturation. Although the majority of these studies have been performed with females, there are some studies which have included male gymnasts showing similar results (Gruodyte-Raciene et al., 2013; Zanker et al., 2003).
Discussion
Differences in bone mineral values between pre-pubertal gymnasts and untrained controls
The results of female pre-pubertal RG (Jürimäe et al., 2016; Parm et al., 2011b) and AG (Cassell et al., 1996; Courteix et al., 1998; Dowthwaite et al., 2006; Greene et al., 2012; Nickols-Richardson et al., 1999; Zanker et al., 2003) bone density measurements by DXA indicated that gymnasts had denser bones compared with UC. The measured aBMD values were higher in RG (WB aBMD: 3.4-4.8%; LS aBMD: 8.7-13.3%; FN aBMD: 9.0-14.5%) and AG (WB aBMD: 4.8-7.4%; LS aBMD: 8.1-17.9%; FN aBMD: 10.2-19.5%; FA aBMD: 10.5-15.5%) in comparison with UC as demonstrated by the results of the included studies in Tables 1 and 2. Even one year of specific gymnastics training had favorable effect on WB aBMD in pre-pubertal female gymnasts (Cassell et al., 1996). In contrast, pre-pubertal male gymnasts with at least one year of training history presented similar WB and LS aBMD values with UC (Zanker et al., 2003). This may demonstrate a possible gender effect in the pre-pubertal years, although the training history in female AG (3-4 yrs) was longer compared to male AG (1-2 yrs) of the same age (Zanker et al., 2003). Similarly, Lehtonen-Veromaa et al. (2000a) showed that pre-pubertal female AG had no differences in bone mineral measures compared to UC, while pubertal AG presented higher LS aBMD (by 9.4%) and FN aBMD (by 15.2%) values compared with UC. Thus, the history of specific gymnastics training appears to be an important parameter in bone development starting in the pre-pubertal years. In addition, as not all measured bone mineral values at different skeletal regions were higher in gymnasts, a site-specific effect of mechanical loading is suggested, again starting in the pre-pubertal years (Burt et al., 2013). Pre-pubertal AG had higher FN aBMD (by 10.2-15.0%) and FA aBMD (by 10.5-15.5%) values compared with age-matched UC (Courteix et al., 1998; Dowthwaite et al., 2006). The only study using three-dimensional pQCT demonstrated higher volumetric BMD at the distal radius by 19.6% in pre-pubertal AG (Cassell et al., 1996). It has been suggested that the gymnastics-specific nature of high-impact loading directly to the distal radius promotes gain in vBMD and higher geometric properties in this specific region of the skeleton, which in turn improves fracture resistance in pre-pubertal female AG (Dowthwaite et al., 2011). Furthermore, positive effect of gymnastics training on the skeleton is more pronounced in cortical bone compared with trabecular bone ((Tournis et al., 2010; Ward et al., 2005). Accordingly, skeletal differences between gymnasts and UC appear to be site specific and start pre-puberty.
Differences in bone mineral values between gymnasts and untrained controls during peri-puberty
Most studies that have investigated bone mineral parameters at different skeletal regions using DXA in RG (Maimoun et al., 2013a; 2013b; Vicente-Rodriguez et al., 2007; Võsoberg et al., 2016) and AG (Dowthwaite et al., 2006; 2011; Erlandson et al., 2012a; Greene et al., 2012; Lehtonen-Veromaa et al., 2000a; Maimoun et al., 2011; 2013a; Pikkarinen et al., 2009; Vicente-Rodriguez et al., 2007) have been performed in peri-pubertal girls with different maturation levels, where studied groups have been composed of gymnasts ranging from Tanner stages I-V. As pubertal maturation has a significant effect on bone mineral acquisition (Maimoun et al., 2014), pre-pubertal and peri-pubertal gymnasts should be studied in separate groups. Nevertheless, peri-pubertal RG (WB aBMD: 3.2-4.6%; LS aBMD: 3.7-9.9%; FN aBMD: 11.9-25.5%) and AG (WB aBMD: 8.8-11.7%; LS aBMD: 8.4-13.6%; FN aBMD: 9.7-18.2%; FA aBMD: 10.2-13.6%) demonstrated higher bone mineral values at different regions of the skeleton when compared with UC (Tables 1 and 2). These results clearly suggest that specific high-impact gymnastics activity has an influence on bone mineral acquisition in maturing gymnasts. However, it has been suggested that while high-impact gymnastics activity has a favorable effect on aBMD and bone geometry during the whole growth period, bone health benefits seem to be more marked after menarche (Maimoun et al., 2013a), a late peri-pubertal event. While there are no studies performed with solely peri-pubertal AG, the only two studies with peri-pubertal RG have also demonstrated that aBMD values are higher in gymnasts at specific regions of the skeleton (Gruodyte et al., 2010a; Munoz et al., 2004). For example, Gruodyte et al. (2010a) found that peri-pubertal RG had higher LS aBMD by 3.7% and FN aBMD by 11.9% in comparison with UC. Therefore, a site-specific effect of mechanical loading was suggested by Munoz et al. (2004), where RG had higher FN aBMD by 14.4% and lower FA aBMD by 13.3% in comparison with UC. These results demonstrate that while lower extremities are highly affected by specific rhythmic gymnastics training, there is not enough mechanical loading for upper extremities to produce additional bone mineralization in highly-trained RG whose training volume is higher than 20 h/week (Maimoun et al., 2013b). This is in contrast with AG, where upper extremities are heavily involved in gymnastics trainings. Accordingly, results from the pQCT analyses demonstrated that peri-pubertal AG displayed greater bone strength index at the distal (+157.3 %) and proximal (+83.2 %) radius because of greater BMC, larger total bone cross-sectional area, and higher trabecular and cortical vBMD (Maimoun et al., 2011). Additional advantages to the AG included a larger muscle cross-sectional area at the proximal forearm (34.8%) than UC girls (Maimoun et al., 2011). In addition, peri-pubertal RG (Tournis et al., 2010) and AG (Greene et al., 2012) had higher vBMD at the distal tibia compared with UC. Further studies are needed to investigate volumetric bone mineral values in homogeneous groups of peri-pubertal gymnasts at different maturation levels using more sophisticated three-dimensional high resolution pQCT and UC who are also late maturers to better understand the effect of site-specific gymnastics athletic activity on bone development.
Differences in bone mineral values between gymnasts according to participation level
Separate analysis of RG and AG studies based on participation level and training volume (elite, sub-elite and recreational) may suggest that elite gymnasts have higher bone properties than sub-elite and recreational gymnasts. Participation level of gymnasts could be used to describe aBMD values in growing gymnasts, elite gymnasts having greater overall aBMD than non-gymnasts, while recreational gymnasts present similar aBMD with UC (Burt et al., 2013). Elite gymnasts train about 40 h/week (Roupas et al., 2014), sub-elite gymnasts about 15-20 h/week (Dowthwaite et al., 2006; Nurmi-Lawton et al., 2004) and recreational gymnasts less than 10 h/week (Gruodyte-Racience et al., 2013). Differences between bone mineral values according to participation level are not surprising as elite gymnasts have longer training history and are exposed to higher impact forces in addition to higher weekly training volume when compared with less trained and lower participation level gymnasts (Burt et al., 2013). These results suggest that specific gymnastics athletic activity has a positive influence on bone mineral acquisition in growing and maturing gymnasts. However, it has to be taken into account that the positive skeletal benefits that are associated with gymnastics athletic activity may come at a cost, as participation in elite gymnastics could be associated with a potential negative energy balance and psychological pressure when intensive training schedules excess 30 h/week (Burt et al., 2013).
Bone metabolism markers in gymnasts
The effect of gymnastics activity on bone development may also be evaluated by the analysis of specific bone turnover markers (Maimoun et al., 2014). Typically, bone formation and resorption markers are higher after the initiation of puberty and decline in later puberty (Jürimäe et al., 2009; Vaitkeviciute et al., 2016). Negative associations between bone turnover markers and aBMD have been found during peri-puberty (Jürimäe et al., 2009; Vaitkeviciute et al., 2016), and low concentrations of bone formation and resorption markers predict increased aBMD during the development of peak skeletal mass in UC (Slemenda et al., 1997). In contrast, Lehtonen-Veromaa et al. (2000b) showed that there was no difference between bone turnover markers in gymnasts and UC. Bone formation and resorption markers were more related to increases at the LS aBMD and only partially at the FN aBMD of the growing skeleton. This may have been due to the higher biological activity of trabecular bone than that of cortical bone, as LS aBMD consists mostly of trabecular bone (Lehtonen-Veromaa et al., 2000b). Therefore, LS aBMD appears to be less sensitive to the effects of physical training (Maimoun et al., 2014), while FN aBMD is directly affected by the mechanical loading of gymnastics activity (Võsoberg et al., 2017). In their review article, Maimoun et al. (2014) concluded that generally bone turnover markers appear not to be affected, or are only slightly affected, by sports training, because changes in bone turnover markers induced by growth exceed those related to athletic activity.
Similar levels of bone formation markers such as osteocalcin (OC) (Lehtonen-Veromaa et al., 2000b; Maimoun et al., 2013a; 2013b; Nickols-Richardson et al., 1999), bone alkaline phosphatase (Maimoun et al., 2013a; Roupas et al., 2014), procollagen type I N-terminal propeptide (PINP) (Lehtonen-Veromaa et al., 2000b; Maimoun et al., 2013a; 2013b; Tournis et al., 2010), and similar reduction with age and maturity (Maimoun et al., 2014) have been observed in growing gymnasts and UC. However, one study demonstrated that OC and PINP may be higher in AG compared with UC in the post-pubertal period, when bone remodeling tends to decrease (Maimoun et al., 2011). It is thought that bone formation markers are noticeably affected by gymnastics activity only in the context of reduced bone remodeling in advanced pubertal stages (Maimoun et al., 2011; Munoz et al., 2004). Specifically, a higher bone turnover caused by higher bone formation could also partly be related to higher bone mineral gain in post-menarcheal gymnasts, a late pubertal event (Courteix et al., 2007; Maimoun et al., 2011). In accordance, lower values of PINP have been observed in adolescent amenorrheic athletes, which is associated with lower WB and LS aBMD when compared with normally menstruating adolescent athletes (Maimoun et al., 2014; 2016). These results suggest that athletic activity in growing and maturing gymnasts does not affect bone formation markers and/or changes in bone formation markers induced by maturation mask the influence of specific athletic activity. Further longitudinal studies in gymnasts during maturation are needed before any conclusions can be drawn. It has been suggested that more than one bone formation marker should be measured to better monitor bone modeling and remodeling during growth and maturation (Gomez-Bruton et al., 2013; Jürimäe, 2010).
Regarding bone resorption markers, C-terminal cross-linked telopeptide (CTX) (Courtiex et al., 2007; Maimoun et al., 2013a; 2013b; Pikkarinen et al., 2009; Slemenda et al., 1997; Sööt et al., 2005; Vicente-Rodriguez et al., 2007) has been the most studied bone turnover marker in gymnasts, typically demonstrating no difference between gymnasts and UC. Similar reductions in CTX between AG and UC have been observed with increasing age and maturation (Maimoun et al., 2013b). In contrast, CTX has been reported to be higher in post-menarcheal AG when compared with UC (Maimoun et al., 2011). While Munoz et al. (2004) found that elite adolescent RG had higher CTX/creatinine ratio with an inverse association with lower FA aBMD in comparison with UC. It was suggested that a higher bone resorption observed in elite pubertal RG could explain the lower scores found in the relatively unloaded FA aBMD of these RG (Munoz et al., 2004). In contrast, no differences in other bone resorption markers, such as pyridinoline and deoxypyridinoline were observed between sub-elite pre-pubertal AG and UC (Nickols-Richardson et al., 1999), while sclerostin was higher in sub-elite pre-pubertal RG when compared with UC (Jürimäe et al., 2016). Therefore, the modification of the sclerostin in RG was not associated with aBMD values (Jürimäe et al., 2016). The observed increased bone resorption markers in gymnasts than in UC in some studies (Jürimäe et al., 2016; Maimoun et al., 2011; Munoz et al., 2004) may indicate an intense remodeling process, caused by intense gymnastics training, that does not affect negatively bone mineral acquisition during growth and maturation. In comparison, increased bone resorption markers in adolescent amenorrheic athletes are associated with lower aBMD (Maimoun et al., 2014). Although studies with gymnasts have indicated no associations of bone resorption markers with bone mineral acquisition, further longitudinal studies including different bone resorption markers and imaging techniques which allow to analyze bone structure more precisely such as pQCT are needed before any conclusions can be drawn.
The influence of prolonged gymnastic activity on bone health
As discussed above, growing and maturing gymnasts have higher bone mineral values despite their possible negative energy balance, at some stages of training, when compared with UC. However, elite gymnastics is a high-level athletic activity and participation is limited to only a select number of skilled athletes (Erlandson et al., 2012a). It has been suggested that recreational gymnastics is attainable by most children and does not require a high level of training (Erlandson et al., 2011a; 2012a). Laing et al. (2005) were the first to demonstrate that 4- to 8-year-old girls participating in only one hour of recreational gymnastics per week gained more LS aBMD and FA BA over a two year period than girls participating in non-gymnastics activities. Furthermore, when analyzing the relationship between exposure to early childhood recreational gymnastics with bone measures and bone strength development in the same cohort of active children, significantly greater total BA and total BMC at the distal radius were observed in children who participated in recreational gymnastics comparing to their physically active counterparts (Jackowski et al., 2015). While controlling for age, limb length, weight, physical activity, muscle area, sex, and hours of training, skeletal benefits were estimated to be of 8–21 % in total BA and total BMC at the distal radius (Jackowski et al., 2015). In another study, Erlandson et al. (2011b) found that 4- to 6-year-old children presented higher WB and FN BMC after four years of recreational gymnastics training in comparison with UC, who participated in other recreational sport programs. Recreational gymnasts participated approximately 1.5 h/week in gymnastics during the first year and the mean training volume was 4.6 h/week at the fourth year of measurement. However, the response to recreational gymnastics activities was lower as measured WB and FN BMC were lower when compared with the values obtained in sub-elite gymnasts of the same age (Erlandson et al., 2012b). This lower-magnitude response in recreational gymnasts compared to sub-elite gymnasts was not unexpected, as a dose-response relationship between gymnastics exposure (ie, hours and years of training) and bone mass has been suggested (Erlandson et al., 2011b; Georgopoulos et al., 2004; Laing et al., 2005). Despite this, beginning-level gymnastics skills performed in introductory classes of these recreational precompetitive gymnasts seem to be an adequate stimuli for enhancing gains in BMC in the early years (Erlandson et al., 2011a,b; Laing et al., 2005). These findings are important and demonstrate that beginner gymnastics skills are attainable by most children and low-level gymnastics skills can be implemented easily into school physical education programs to promote bone health for life (Erlandson et al., 2011b).
Whether the advantage of a regular gymnastics activity during childhood and adolescence is maintained through the adult years or affects later fracture risk is not still entirely clear (Georgopoulos et al., 2004). It has been suggested that the benefits of gymnastics activities during childhood and adolescence may be retained into adulthood (Erlandson et al., 2012a; 2012b; Eser et al., 2009; Gruodyte-Racience et al., 2013). Specifically, skeletal benefits of gymnastics training in pre-menarcheal AG were maintained 10 years after retirement from gymnastics trainings (Erlandson et al., 2012a). Skeletal adaptations of former female gymnasts were significantly better in geometric and densitometric properties, as well as estimated strength at the radius and tibia compared to females who did not participate in gymnastics in childhood (Erlandson et al., 2012b). It was found that retired gymnasts present 22% to 32% greater estimated bone strength at the radius and 24% greater estimated bone strength at the distal tibia compared to UC (Erlandson et al., 2012b).
Similarly, skeletal advantages of rhythmic gymnastics training persisted at least two years after loading cessation in pre-menarcheal RG (Scerpella et al., 2010). Scerpella et al. (2011) suggested that skeletal benefits appear to persist at least four years beyond of rhythmic gymnastics activity cessation into early adulthood. These findings support the notion that structured athletic activity of gymnastics training during growth and maturation is an effective tool to increase bone mineral values, as well as structural strength, that persist into adulthood even up to 10 years after retiring from the sport and potentially prevent the risk of osteoporosis and related fracture in later life (Erlandson et al., 2012b). However, long-term prospective studies of retired gymnasts, especially when bone loss accelerates (eg, in females at the time of menopause) are required to determine the impact on fracture risk (Erlandson et al., 2012b).
Factors influencing bone mineral density in gymnasts during growth
Athletic training
Gymnastics activity has been demonstrated to have direct osteogenic effect on bone cell activity via mechanical loading (Dowthwaite et al., 2012; Gruodyte-Racience et al., 2013). It appears that gymnastics activity constitutes a specific type of exercises, with intense mechanical load on the skeleton, that exert a beneficial effect on bone mineral accrual in growing athletes, despite possible negative energy balance and estrogen deficiency (Maimoun et al., 2014; Markou et al., 2010). It has been found that elite gymnastics training in peri-pubertal AG is characterized with on average 102 and 217 impacts per session on the upper and lower extremeties, respectively (Daly et al., 1999). The magnitudes of such impacts peaked at 3.6 and 10.4 times body mass and were associated with a high rate of loading (ie, rapid rise to peak vertical force) (Daly et al., 1999). Accordingly, skeletal adaptations to mechanical loading in gymnasts have site-specific impact through higher muscle mass and strength, as muscle and bone are biomechanically linked (Daly et al., 1999; Dowthwaite et al., 2012). In addition, estrogen deficiency appears to have a higher negative influence on trabecular bone and skeletal site receiving low mechanical loads (eg, LS aBMD). Whereas exercise compensates more negative estrogen effects at mechanically loaded cortical bone sites (eg, FN aBMD) that are also more influenced by body composition factors in growing athletes (Maimoun et al., 2014).
In addition to a specific exercise type, early age at initiation and weekly training volume may influence bone development in growing and maturing gymnasts (Malina et al., 2013). However, this information was not always available in gymnasts discussed in this review. Typically, elite gymnastics training is characterized by a very high volume, as the mean weekly training volume of elite female RG participating in the World Championships was about 41 h/week and the mean onset of training was 6 years of age (Roupas et al., 2014). In comparison, AG enter sport at 4-6 years of age and average training time reported by AG at major championships was about 30 h/week (Malina et al., 2013). It has also been demonstrated that years of gymnastics training showed positive association with bone mineral values independent of chronological age in elite pre-menarcheal RG (Maimoun et al., 2013b). It appears that the osteogenic effect of gymnastics athletic activity has high positive influence on bone development and overcomes possible negative influence of high training volume that may cause negative energy balance in elite gymnasts during growth and maturation.
Age and pubertal maturation
Gymnastics training constitutes a metabolic model prone to develop menstrual irregularities or late menarche and concomitant estrogen deficiency (Roupas et al., 2014), which has been associated with a deficiency in peak bone accrual (Maimoun et al., 2014,2016). Late puberty possibly caused by intensive physical training has been reported in elite RG and AG (Georgopoulos et al., 2010). Pre-pubertal stage could be prolonged and pubertal development shifted to a later age in elite gymnasts, maintaining a normal rate of pubertal progression as normal girls require an average about two years for their breast development to progress from Tanner stage 2 to Tanner stage 4 (Daly et al., 1999; Dowthwaite et al., 2012). The progression of puberty follows bone age rather than chronological age in elite gymnasts (Theodoropoulou et al., 2005). Later skeletal maturation, which is the difference between chronological age and bone age could be about 1-3 years in gymnasts (Maimoun et al., 2010a; 2010b; Munoz et al., 2004), and is correlated with an energy deficit (Maimoun et al., 2014). However, negative impact of intensive physical training on growth velocity and sexual maturation is generally observed only in gymnasts whose mean weekly training volume exceeds 15 h, as recreational gymnasts present natural growth and maturation pattern (Erlandson et al., 2008). Bone mineral accrual is proportional to the development of puberty according to pubertal stages of breast development in female gymnasts, and there appears to be a negative influence of early onset of training and training intensity on pubertal maturation and consequently on bone acquisition, which is shifted to a later age in elite gymnasts (Georgopoulos et al., 2010).
Body composition
Intense athletic activity, early age at initiation and aesthetic appeal that requires strict weight control with low FM are characteristics in elite gymnastics (Misra, 2008; Võsoberg et al., 2014,2017). Already pre-pubertal RG have lower FM when compared with UC (Parm et al., 2011a; 2011b), while no difference in fat free mass (FFM) has been observed between RG and UC entering puberty (Võsoberg et al., 2016,2017). Prolonged gymnastics activity in childhood can lead to a state of energy deficiency, which can lower FM in growing athletes (Võsoberg et al., 2014). A decreased FM, together with low leptin, has been implicated as a cause of hypothalamic amenorrhea in adolescent athletes (Misra, 2008), and reduced aBMD is characteristic of adolescent amenorrheic athletes (Maimoun et al., 2014). FM and FFM are positively associated with aBMD during growth and pubertal development in girls with different physical activity and body composition values (Gomez-Bruton et al., 2016; Gruodyte et al., 2010a; Maimoun et al., 2010b) and both body composition compartments are also positively correlated with aBMD in pre-pubertal RG with already lowered FM (Parm et al., 2011b). However, only FFM is associated with increases in bone mineral values in RG with relatively low FM entering puberty (Võsoberg et al., 2016,2017). Typically, FFM is a better determinant of bone mineral acquisition in normal weight children, while FM is a better predictor of aBMD in overweight children (Ivuskans et al., 2015). A positive influence of FM on bone mineral acquisition has been attributed to a combination of mechanical loading (Reid, 2002) and the impact of several hormones linked to adipose tissue (Võsoberg et al., 2016). Võsoberg et al. (2016) concluded that high-intensity gymnastics training appears to increase bone mineral acquisition and counterbalance negative effects of slower pubertal development, low FM and low leptin values in RG entering puberty.
Hormonal profile
It is well known that various hormones that affect bone metabolism and consequently bone mineral acquisition, change during growth and maturation. As already stated, gymnastics activity has a direct effect on bone mineral acquisition via mechanical loading (Gruodyte-Racience et al., 2013), while an indirect effect is generated via hormonal regulation in growing and maturing athletes (Maimoun et al., 2014). This hormonal effect can be positive or negative. For example, estrogens together with growth hormone (GH) and insulin-like growth factor-I (IGF-I) are important bone trophic hormones, and are important for pubertal bone modeling (Misra, 2008). Increasing levels of these hormones are responsible for the attainment of maximal peak bone mass acquisition during maturation in athletes (Markou et al., 2010). In contrast, heavy gymnastics activity in states of decreased energy balance has been suggested to exert an inhibitory effect on sex hormones in female gymnasts (Malina et al., 2013), and hypoestrogenism negatively influences bone development by increasing bone resorption and decreasing bone formation markers in amenorrheic adolescent athletes (Misra, 2008). Late menarche and concomitant estrogen deficiency in untrained adolescents have been related to lower peak bone accrual (Jackowski et al., 2011a; Maimoun et al., 2014), which demonstrates that the exposure to estrogen is an important factor for bone mineral acquisition in gaining maximal peak bone accrual (Chevalley et al., 2011).
No significant differences in estradiol were found between pubertal RG and UC, although estradiol levels were slightly lower in gymnasts (Gruodyte et al., 2010b). There are different studies to show positive associations between estradiol or testosterone levels with different aBMD and BMC values in RG during puberty (Gruodyte et al., 2010b; Maimoun et al., 2014), which provides a strong confirmation of the involvement of these sex hormones in bone mass accretion (Maimoun et al., 2014). However, no relationships between estradiol and bone mineral values have been observed among other pubertal athlete groups (Gruodyte et al., 2010b), which is similar to the results of other studies with adult female athletes of different sport disciplines (Sööt et al., 2006). It is suggested that lower estradiol values caused by intense athletic activity may be compensated by engaging in frequent high-impact loading (Gruodyte et al., 2010b). The adaptive response of bone cells to mechanical loading involves estrogen receptor and blocking estrogen receptor impairs bone formation response to mechanical strain (Zaman et al., 2000). These results suggest that although estradiol is positively associated with bone mass accretion in gymnasts during pubertal maturation, estradiol has only modest indirect effect and specific high-impact mechanical loading is a more important parameter in bone development during puberty in gymnasts.
Rising levels of estrogen during puberty are closely followed by rising GH and IGF-I levels, which both positively affect bone turnover by stimulating osteoblast proliferation and differentiation (Davies et al., 2005). Estradiol has been reported to correlate with IGF-I and IGF-binding protein-3 (IGFBP-3) in adolescent RG (Gruodyte et al., 2010b). Therefore, pubertal RG presented similar IGF-I and IGFBP-3 values with UC (Gruodyte et al., 2010b). The associations of estradiol with serum IGF-I and IGF-I/IGFBP-3 ratio have also been revealed in UC with different pubertal maturation levels (Kanbur-Öksüz et al., 2004). Correlations of IGF-I and IGF-I/IGFBP-3 ratio with FN and LS aBMD and BMC values have been found in pubertal RG (Gruodyte et al., 2010b), while IGF-1/IGFBP-3 ratio was strongly correlated with aBMD gain over 1-year in peripubertal RG (Maimoun et al., 2010a). The associations between IGF-I and IGF-I/IGFBP-3 ratio and bone mass acquisition in pubertal RG indicate that the indices of IGF-I axis may serve as surrogate markers of bone mineral gain in gymnasts during puberty (Maimoun et al., 2010a).
It appears that nutritional level, and more specifically energy balance, has a great influence on linear growth and pubertal maturation through the influence of leptin in athletes (Jürimäe, 2014). In general, in the presence of elevated energy expenditure, chronic athletic activity decreases leptin levels in athletes (Jürimäe et al., 2011). Leptin is positively correlated with FM and aBMD values in healthy lean UC girls with different maturation levels (Garnett et al., 2004; Parm et al., 2011b). The impact of lowered leptin on bone mineral acquisition in the presence of elevated energy expenditure and reduced FM remains questionable in pre-pubertal (Parm et al., 2011b; 2012) and pubertal (Courteix et al., 2007; Maimoun et al., 2010b) RG. However, two studies have reported positive correlations between leptin and bone mineral values in pubertal RG (Gruodyte et al., 2010a; Munoz et al., 2004). Other investigations did not find leptin as a predictor of aBMD gain in pre-pubertal (Parm et al., 2012) and pubertal (Maimoun et al., 2010b) RG. Furthermore, Courteix et al. (2007) found that leptin levels in elite adolescent RG were as low as those observed in anorectic subjects, while aBMD values were greater in gymnasts than in controls, and concluded that heavy gymnastics activity counterbalanced negative effect that leptin deficiency has on bone. In contrast, leptin concentrations were correlated with increases in aBMD and BMC values in UC girls entering puberty (Võsoberg et al., 2016). The effect of leptin on bone development is likely multifactorial and may involve other hormones such as estradiol and IGF-I, in addition to its possible direct actions on bone (Maimoun et al., 2014). Taken together, the impact of leptin on bone mineral acquisition in growing human bone remains controversial and may depend on the specific chronic athletic activity.
The role of other hormones that may influence bone development during chronic gymnastics activity with high energy expenditure is not fully understood in athletes during growth and maturation. Adiponectin (Võsoberg et al., 2016), visfatin (Gruodyte et al., 2010a), ghrelin (Parm et al., 2011b) and preadipocyte factor-1 (Pref-1) (Jürimäe et al., 2016) among other hormones that participate in the regulation of energy homeostasis have been studied in relation to bone mineral acquisition in growing and maturing gymnasts. These hormone levels appear to be higher in growing and maturing RG in comparison with age-matched UC (Gruodyte et al., 2010a; Jürimäe et al., 2016; Parm et al., 2011b; Võsoberg et al., 2016). However, adiponectin and ghrelin did not predict bone mineral mass gain in RG entering puberty (Võsoberg et al., 2016). Adiponectin and visfatin were not correlated with aBMD and BMC in pubertal RG (Gruodyte et al., 2010a), and increased Pref-1 was not associated with increased bone mineral values in pre-pubertal RG (Jürimäe et al., 2016). In agreement with these results, adiponectin was associated with weekly training volume but not with bone mass acquisition in elite RG participating in World Championships (Roupas et al., 2014). Adiponectin has been suggested as a link between bone and fat metabolism (Donoso et al., 2010), and adiponectin has been found to be a negative predictor of aBMD in healthy untrained adolescent (Misra et al., 2007) and adult (Jürimäe et al., 2005) females. In addition, adiponectin and ghrelin levels predicted increments in measured aBMD values in pre-pubertal UC entering puberty over a 3-year period (Võsoberg et al., 2016). The results with different markers of energy homeostasis suggest that specific gymnastics athletic activity and body composition with reduced FM may modify the possible associations between markers of energy homeostasis and bone mineral accrual in girls during growth and maturation. It appears that the impact of these energy homeostasis markers on bone mineral accrual remains controversial, and these hormones are typically not related to bone mineral gain in growing and maturing gymnasts.
Limitations
The included studies were not all homogeneous with all necessary information included to perform a meta-analysis. In fact, many articles were only of moderate quality. Although Tables 1 and 2 contain information on each cited study, the classification of the articles according to the bone assessment method (DXA and/or pQCT) is not a closed issue. To some degree, aBMD and BMC as measured by radiological methods, represent only static results of gymnastics activity on bone. Bone metabolism markers should also be obtained to more sensitively reflect changes in bone tissue as a result of gymnastics activity. The interactions between growth and specific gymnastics activities should be further studied in a longitudinal design to better understand adult bone health. Finally, current studies regarding the beneficial effects of gymnastics activity on bone mineral gain and better adult bone health are mainly focused on female gymnasts, while such studies in male gymnasts are mostly still lacking.
Conclusion
The findings of prolonged high gymnastics activity during growth and maturation presented in this review would suggest that the systematic practice of gymnastics affects positively bone accrual. Already pre-pubertal gymnasts present higher aBMD and BMC in comparison with untrained controls, who are not participating in any athletic activity and therefore not stimulating their bone tissue to achieve maximum possible peak bone mineral acquisition. Early age at initiation and aesthetic appeal that requires strict weight control with low FM are characteristics in elite gymnastics training and do not affect bone mineral acquisition positively. However, gymnastic activities are characterized by high-impact weight-bearing mechanical loading, which appear to overcome negative influence of other characteristics. Gymnastics training is osteogenic for bone development in children. While regular exercise is critically important to improve and maintain bone health throughout the life, early puberty seems to be the most sensitive period for maximizing bone mineral gain. Specific gymnastics activities are the most effective exercises to improve bone mineral gain in growing and maturing children. Recreational gymnastics is attainable by most children and does not require a high level of training, and already few hours of training per week have a positive influence on bone development.
Acknowledgment
This work was supported by Estonian Ministry of Education and Science Institutional Grant IUT 20-58. The authors have no conflicts of interest to declare. All experiments comply with the current laws of the country.
Biographies
Jaak JÜRIMÄE
Employment
Professor at the Institute of Sport Sciences and Physiotherapy, University of Tartu, Estonia
Degree
PhD
Research interests
Exercise physiology, growth and maturation, body composition, pediatric endocrinology
E-mail: jaak.jurimae@ut.ee
Rita GRUODYTE-RACIENCE
Employment
Associate Professor at the Department of Health, Physical and Social Education, Lithuanian Sports University, Lithuania
Degree
PhD
Research interests
Physical activity and health, kinanthropometry, pediatric endocrinology
E-mail: rita.gruodyte@lsu.lt
Adam D. G. BAXTER-JONES
Employment
Professor at the College of Kinesiology, University of Saskatchewan, Canada
Degree
PhD
Research interests
Bone development, growth and maturation, body composition, exercise physiology
E-mail: baxter.jones@usask.ca
References
- Bailey D.A., McKay H.A., Mirwald R.L., Crocker P.R., Faulkner R.A. (1999) A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan Bone Mineral Accrual Study. Journal of Bone and Mineral Research 14, 1672-1679. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones A.D.G., Kontulainen S.A., Faulkner R.A., Bailey D.A. (2008) A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 43, 1101-1107. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones A.D.G., Faulkner R.A, Forwood M., Mirwald R.L., Bailey D.A. (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. Journal of Bone and Mineral Research 26, 1729-1739. [DOI] [PubMed] [Google Scholar]
- Beck T.J., Ruff C.B., Warden K.E., Scott W.W., Jr., Rao G.V. (1990) Predicting femoral neck strength from bone mineral data. A structural approach. Investigative Radiology 25, 6-18. [DOI] [PubMed] [Google Scholar]
- Burt L.A., Naughton G.A., Greene D.A., Courtiex D., Ducher G. (2012) Non-elite gymnastics participation is associated with greater bone strength, muscle size, and function in pre- and early pubertal girls. Osteoporosis International 23, 1277-1286. [DOI] [PubMed] [Google Scholar]
- Burt L.A., Greene D.A., Ducher G., Naughton G.A. (2013) Skeletal adaptations associated with pre-pubertal gymnastics participation as determined by DXA and pQCT: a systematic review and meta-analysis. Journal of Science and Medicine in Sport 16, 231-239. [DOI] [PubMed] [Google Scholar]
- Cassell C., Benedict M., Specker B. (1996) Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Medicine and Science in Sports and Exercise 28, 1243-1246. [DOI] [PubMed] [Google Scholar]
- Chevalley T., Bonjour J.P., Ferrari S., Rizzoli R. (2011) Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporosis International 22, 2689-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courteix D., Lespessailles E., Peres S.L., Obert P., Ferry B., Benhamou C.L. (1998) Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporosis International 8, 152-158. [DOI] [PubMed] [Google Scholar]
- Courteix D., Rieth N., Thomas T., van Praagh E., Benhamou C.L., Collomp K, Lespessailles E., Jaffre C. (2007) Preserved bone health in adolescent elite rhythmic gymnasts despite hypoleptinemia. Hormone Research 68, 20-27. [DOI] [PubMed] [Google Scholar]
- Daly R.M., Rich P.A., Klein R., Bass S. (1999) Effects of high-impact exercise on ultrasonic and biochemical indicies of skeletal status: a prospective study in young male gymnasts. Journal of Bone and Mineral Research 14, 1222-1230. [DOI] [PubMed] [Google Scholar]
- Davies J.H., Evans B.A.J., Gregory J.W. (2005) Bone mass acquisition in healthy children. Archives of Disease in Childhood 90, 373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso M.A., Munoz-Calvo M.T., Barrios V., Garrido G., Hawkins F., Argente J. (2010) Increased circulating adiponectin levels and decreased leptin/soluble leptin receptor ratio throughout puberty in female ballet dancers: associations with body composition and the delay in puberty. European Journal of Endocrinology 162, 905-911. [DOI] [PubMed] [Google Scholar]
- Dowthwaite J.N., DiStefano J.G., Ploutz-Snyder R.J., Kanaley J.A., Scerpella T.A. (2006) Maturity and activity-related differences in bone mineral density: Tanner I vs. II and gymnasts vs. non-gymnasts. Bone 39, 895-900. [DOI] [PubMed] [Google Scholar]
- Dowthwaite J.N., Rosenbaum P.F., Scerpella T.A. (2011) Mechanical loading during growth is associated with plane-specific differences in vertebral geometry: a cross-sectional analysis comparing artistic gymnasts vs. non-gymnasts. Bone 49, 1046-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowthwaite J.N., Rosenbaum P.F., Scerpella T.A. (2012) Site-specific advantages in skeletal geometry and strength at the proximal femur and forearm in young female gymnasts. Bone 50, 1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson K., Blimkie C.J., Davison K.S., Webber C.E., Adarchi J.D. (1997) Gymnastics training and bone density in pre-adolescent females. Medicine and Science in Sports and Exercise 29, 443-450. [DOI] [PubMed] [Google Scholar]
- Erlandson M.C., Sherar L.B., Mirwald R.L., Maffulli N., Baxter-Jones A.D. (2008) Growth and maturation of adolescent female gymnasts, swimmers, and tennis players. Medicine and Science in Sports and Exercise 40, 34-42. [DOI] [PubMed] [Google Scholar]
- Erlandson M.C., Kontulainen S.A., Baxter-Jones A.D. (2011a) Precompetitive and recreational gymnasts have greater bone density, mass, and estimated strength at the distal radius in young childhood. Osteoporosis International 22, 75-84. [DOI] [PubMed] [Google Scholar]
- Erlandson M.C., Kontulainen S.A., Chilibeck P.D., Arnold C.M., Baxter-Jones AD. (2011b) Bone mineral accrual in 4- to 10-year-old precompetitive, recreational gymnasts: a 4-year longitudinal study. Journal of Bone and Mineral Research 26, 1313-1320. [DOI] [PubMed] [Google Scholar]
- Erlandson M.C., Kontulainen S.A., Chilibeck P.D., Arnold C.M., Faulkner R.A., Baxter-Jones A.D. (2012a) Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: a 14-year follow-up. Journal of Bone and Mineral Research 27, 104-110. [DOI] [PubMed] [Google Scholar]
- Erlandson M.C., Kontulainen S.A., Chilibeck P.D., Arnold C.M., Faulkner R.A., Baxter-Jones A.D. (2012b) Former premenarcheal gymnasts exhibit site-specific skeletal benefits in adulthood after long-term retirement. Journal of Bone and Mineral Research 27, 2298-2305. [DOI] [PubMed] [Google Scholar]
- Eser P., Hill B., Ducher G., Bass S. (2009) Skeletal benefits after long-term retirement in former elite female gymnasts. Journal of Bone and Mineral Research 24, 1981-1988. [DOI] [PubMed] [Google Scholar]
- Garnett S.P., Högler W., Blades B., Baur L.A., Peat J., Lee J., Lee J., Cowell CT. (2004) Relation between hormones and body composition, including bone, in prepubertal children. American Journal of Clinical Nutrition 80, 966-972. [DOI] [PubMed] [Google Scholar]
- Georgopoulos N.A., Markou K., Theodoropoulou A., Vagenakis G.A., Mylonas P., Vagenakis A.G. (2004) Growth, pubertal development, skeletal maturation and bone mass acquisition in athletes. Hormones 3, 233-243. [DOI] [PubMed] [Google Scholar]
- Georgopoulos N.A., Roupas N.D., Theodoropoulou A., Tsekouras A., Vagenakis A.G., Markou K.B. (2010) The influence of intensive physical training on growth and pubertal development in athletes. Annals of New York Academy of Sciences 1205, 39-44. [DOI] [PubMed] [Google Scholar]
- Gomez-Bruton A., Gonzalez-Agüero A., Gomez-Cabello A., Vicente-Rodriguez G. (2013) Is bone tissue really affected by swimming? A systematic review. Plos One 8, e70119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Bruton A., Montero-Marin J., Gonzalez-Agüero A., Garcia-Campayo J., Moreno L.A., Casajus J.A., Vicente-Rodriguez G. (2016) The effect of swimming during childhood and adolescence on bone mineral density: a systematic review and meta-analysis. Sports Medicine 46, 365-379. [DOI] [PubMed] [Google Scholar]
- Greene D.A., Naughton G.A., Bradshaw E., Moresi M., Ducher G. (2012) Mechanical loading with or without weight-bearing activity: influence on bone strength index in elite female adolescent athletes engaged in water polo, gymnastics, and track-and-field. Journal of Bone and Mineral Metabolism 30, 580-587. [DOI] [PubMed] [Google Scholar]
- Gruodyte R., Jürimäe J., Saar M., Maasalu K., Jürimäe T. (2009) Relationships between areal bone mineral density and jumping height in pubertal girls with different physical activity patterns. Journal of Sports Medicine and Physical Fitness 49, 474-479. [PubMed] [Google Scholar]
- Gruodyte R., Jürimäe J., Cicchella A., Stefanelli C., Passariello C., Jürimäe T. (2010a) Adipocytokine and bone mineral density in adolescent female athletes. Acta Paediatrica 99, 1879-1884. [DOI] [PubMed] [Google Scholar]
- Gruodyte R., Jürimäe J., Saar M., Jürimäe T. (2010b) The relationship among bone health, insulin-like growth factor-1 and sex hormones in adolescent female athletes. Journal of Bone and Mineral Metabolism 28, 306-313. [DOI] [PubMed] [Google Scholar]
- Gruodyte-Raciene R., Erlandson M.C., Jackowski S.A., Baxter-Jones A.D. (2013) Structural strength development at the proximal femur in 4- to 10-year-old precompetitive gymnasts: a 4-year longitudinal hip structural analysis study. Journal of Bone and Mineral Research 28, 2592-2600. [DOI] [PubMed] [Google Scholar]
- Ivuskans A., Lätt E., Mäestu J., Saar M., Purge P., Maasalu K, Jürimäe T., Jürimäe J. (2013) Bone mineral density in 11-13-year-old boys: relative importance of the weight status and body composition factors. Rheumatology International 33, 1681-1687. [DOI] [PubMed] [Google Scholar]
- Ivuskans A, Mäestu J, Jürimäe T, Lätt E, Purge P, Saar M, Maasalu K., Jürimäe J. (2015) Sedentary time has a negative influence on bone mineral parameters in peripubertal boys: one-year prospective study. Journal of Bone and Mineral Metabolism 33, 85-92. [DOI] [PubMed] [Google Scholar]
- Jackowski S.A., Faulkner R.A., Farthing J.P., Kontulainen S.A., Beck J.J., Baxter-Jones A.D. (2009) Peak lean tissue mass accrual precedes changes in bone strength indices at the proximal femur during the pubertal growth spurt. Bone 44, 1186-1190. [DOI] [PubMed] [Google Scholar]
- Jackowski S.A., Erlandson M.C., Mirwald R.L., Faulkner J.A., Bailey D.A., Kontulainen S.A., Cooper D.M., Baxter-Jones A.D. (2011a) Effect of maturational timing on bone mineral content accrual from childhood to adulthood: evidence from 15 years of longitudinal data. Bone 48, 1178-1185. [DOI] [PubMed] [Google Scholar]
- Jackowski S.A., Kontulainen S.A., Cooper D.M., Lanovaz J.L., Baxter-Jones A.D. (2011b) The timing of BMD and geometric adaptation at the proximal femur from childhood to early adult hood in males and females: a longitudinal study. Journal of Bone and Mineral Research 26, 2753-2761. [DOI] [PubMed] [Google Scholar]
- Jackowski S.A., Baxter-Jones A.D.G., Gruodyte-Raciene R., Kontulainen S.A., Erlandson M.C. (2015) A longitudinal study of bone area, content, density, and strength development at the radius and tibia in children 4–12 years of age exposed to recreational gymnastics. Osteoporosis International 26, 1677-1690. [DOI] [PubMed] [Google Scholar]
- Jürimäe J., Rembel K., Jürimäe T., Rehand M. (2005) Adiponectin is associated with bone mineral density in perimenopausal women. Hormone and Metabolic Research 37, 297-302. [DOI] [PubMed] [Google Scholar]
- Jürimäe J., Pomerants T., Tillmann V., Jürimäe T. (2009) Bone metabolism markers and ghrelin in boys at different stages of sexual maturity. Acta Paediatrica 98, 892-896. [DOI] [PubMed] [Google Scholar]
- Jürimäe J. (2010) Interpretation and application of bone turnover markers in children and adolescents. Current Opinion in Pediatrics 22, 494-500. [DOI] [PubMed] [Google Scholar]
- Jürimäe J., Mäestu J., Jürimäe T., Mangus B., von Duvillard S.P. (2011) Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism 60, 335-350. [DOI] [PubMed] [Google Scholar]
- Jürimäe J. (2014) Adipocytokine and ghrelin responses to acute exercise and sport training in children during growth and maturation. Pediatric Exercise Science 26 392-403. [DOI] [PubMed] [Google Scholar]
- Jürimäe J., Tillmann V., Cicchella A., Stefanelli C., Võsoberg K., Tamm A.L, Jürimäe T. (2016) Increased sclerostin and preadipocyte factor-1 levels in prepubertal rhythmic gymnasts: associations with bone mineral density, body composition, and adipocytokine values. Osteoporosis International 27, 1239-1243. [DOI] [PubMed] [Google Scholar]
- Kanbur-Öksüz N., Derman O., Kinik E. (2004) Correlation of sex steroids with IGF-1 and IGFBP-3 during different pubertal stages. Turkish Journal of Pediatrics 46, 315-321. [PubMed] [Google Scholar]
- Laing E.M., Wilson A.R., Modlesky C.M., O`Connor P.J., Hall D.B., Lewis R.D. (2005) Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year old females. Journal of Bone and Mineral Research 20, 509-519. [DOI] [PubMed] [Google Scholar]
- Lehtonen-Veromaa M., Möttönen T., Svedström E., Hakola P., Heinonen O.J., Viikari J. (2000a) Physical activity and bone mineral acquisition in peripubertal girls. Scandinavian Journal of Medicine and Science in Sports 10, 236-243. [DOI] [PubMed] [Google Scholar]
- Lehtonen-Veromaa M., Möttönen T., Irjala K., Nuotio I., Leino A., Viikari J. (2000b) A 1-year prospective study on the relationship between physical activity, markers of bone metabolism, and bone acquisition in peripubertal girls. Journal of Clinical Endocrinology and Metabolism 85, 3726-3732. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ionnidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. (2009) PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 62, e1-e34. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Galtier F., Mura T., Mariono-Goulart D., Paris F., Sultan C. (2010) Bone mineral density acquisition in peripubertal female rhythmic gymnasts is directly associated with plasma IGF1/IGF-binding protein 3 ratio. European Journal of Endocrinology 163, 157-164. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Jaussent A., Mariano-Goulart D., Sultan C., Paris F. (2010) Bone mass acquisition in female rhythmic gymnasts during puberty: no direct role for leptin. Clinical Endocrinology 72, 604-611. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Mariano-Goulart D., Gattier F., Mura T., Philibert P., Briot K., Paris F., Sultan C. (2011) In peripubertal girls, artistic gymnastics improves areal bone mineral density and femoral bone geometry without affecting serum OPG/RANKL levels. Osteoporosis International 22, 3055-3066. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Philibert P., Briot K., Mura T., Galtier F., Mariano-Goulart D., Paris F., Sultan C. (2013a) Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism 62, 1088-1098. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Coste O., Mura T., Philibert P., Galtier F., Mariano-Goulart D., Paris F., Sultan C. (2013b) Specific bone mass acquisition in elite female athletes. Journal of Clinical Endocrinology and Metabolism 98, 2844-2853. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Georgopoulos N.A., Sultan C. (2014) Endocrine disorders in adolescent and young female athletes: impact on growth, menstrual cycles, and bone mass acquisition. Journal of Clinical Endocrinology and Metabolism 99, 4037-4050. [DOI] [PubMed] [Google Scholar]
- Maimoun L., Guillaume S., Lefebvre P., Philibert P., Bertet H., Picot M.C., Gaspari L., Paris F., Seneque M., Dupuys A.M., Courtet P., Thomas E., Mariano-Goulart D., Bringer J., Renard E., Sultan C. (2016) Evidence of a link between resting energy expenditure and bone remodelling, glucose homeostasis and adipokine variations in adolescent girls with anorexia nervosa. Osteoporosis International 27, 135-146. [DOI] [PubMed] [Google Scholar]
- Malina R.M., Baxter-Jones A.D.G., Armstrong N., Beunen G.P., Caine D., Daly R.M., Lewis R.D., Rogol A.D., Russell K. (2013) Role of intensive training in the growth and maturation of artistic gymnasts. Sports Medicine 43, 783-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou K.B., Theodoropoulou A., Tsekouras A., Vagenakis A.G., Georgopoulos N.A. (2010) Bone acquisition during adolescence in athletes. Annals of New York Academy of Sciences 1205, 12-16. [DOI] [PubMed] [Google Scholar]
- Misra M., Miller K.K., Cord J., Prabhakeran R., Herzog D.B., Goldstein M., Katzman D.K., Klibanski A. (2007) Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. Journal of Clinical Endocrinology and Metabolism 92, 2046-2052. [DOI] [PubMed] [Google Scholar]
- Misra M. (2008) Bone density in the adolescent athlete. Reviews in Endocrine and Metabolic Disorders 9, 139-144. [DOI] [PubMed] [Google Scholar]
- Muñoz M.T., de la Piedra C., Barrios V., Garrido G., Argente J. (2004) Changes in bone density and bone markers in rhythmic gymnasts and ballet dancers: implications for puberty and leptin levels. European Journal of Endocrinology 151, 491-496. [DOI] [PubMed] [Google Scholar]
- Nickols-Richardson S.M., O`Connor P.J., Shapses S.A., Lewis R.D. (1999) Longitudinal bone mineral density changes in female child artistic gymnasts. Journal of Bone and Mineral Research 14, 994-1002. [DOI] [PubMed] [Google Scholar]
- Nurmi-Lawton J.A., Baxter-Jones A.D., Mirwald R.L., Bishop J.A., Taylor P., Cooper C., New S.A. (2004) Evidence of sustained skeletal benefits from impact-loading exercise in young females: a 3-year longitudinal study. Journal of Bone and Mineral Research 19, 314-322. [DOI] [PubMed] [Google Scholar]
- Olmedillas H., Gonzalez-Aguero A., Moreno L.A., Casajus J.A., Vicente-Rodriguez G. (2012) Cycling and bone health: a systematic review. BMC Medicine 10, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parm A.L., Saar M., Pärna K., Jürimäe J., Maasalu K., Neissaar I., Jürimäe T. (2011a) Relationships between anthropometric, body composition and bone mineral parameters in 7-8-year-old rhythmic gymnasts compared with controls. Collegium Antropologicum 35, 739-745. [PubMed] [Google Scholar]
- Parm A.L., Jürimäe J., Saar M., Pärna K., Tillmann V., Maasalu K., Jürimäe T. (2011b) Plasma adipocytokine and ghrelin levels in relation to bone mineral density in prepubertal rhythmic gymnasts. Journal of Bone and Mineral Metabolism 29, 717-724. [DOI] [PubMed] [Google Scholar]
- Parm A.L., Jürimäe J., Saar M., Pärna K., Tillmann V., Maasalu K., Jürimäe T. (2012) Bone mineralization in rhythmic gymnasts before puberty: no longitudinal associations with adipocytokine and ghrelin levels. Hormone Research in Paediatrics 77, 369-375. [DOI] [PubMed] [Google Scholar]
- Pikkarinen E., Lehtonen-Veromaa M., Kaitiainen H., Heinonen O.J., Vaikari J., Möttonen T. (2009) Exercise-induced training effects on bone mineral content: a 7-year follow-up study with adolescent female gymnasts and runners. Scandinavian Journal of Medicine and Science in Sports 19, 166-173. [DOI] [PubMed] [Google Scholar]
- Pomerants T., Tillmann V., Jürimäe J., Jürimäe T. (2007) The influence of serum ghrelin, IGF axis and testosterone on bone mineral density in boys at different stages of sexual maturity. Journal of Bone and Mineral Metabolism 25, 193-197. [DOI] [PubMed] [Google Scholar]
- Reid I.R. (2002) Relationships among body mass, its compartments, and bone. Bone 31, 547-555. [DOI] [PubMed] [Google Scholar]
- Roupas N.D., Maimomun L., Mamali I., Coste O., Tsouka A., Mahedea K.K., Mura T., Philibert P., Gaspari L., Mariano-Goulart D., Leglise M., Sultan C., Georgopoulos N.A. (2014) Salivary adiponectin levels are associated with training intensity but not with bone mass or reproductive function in elite rhythmic gymnasts. Peptides 51, 80-85. [DOI] [PubMed] [Google Scholar]
- Saggese G., Baroncelli G.I., Bertelloni S. (2001) Osteoporosis in children and adolescents: diagnosis, risk factors, and prevention. Journal of Pediatric Endocrinology and Metabolism 14, 833-859. [DOI] [PubMed] [Google Scholar]
- Scerpella T.A., Dowthwaite J.N., Gero N.M., Kanaley J.A., Ploutz-Snyder R.J. (2010) Skeletal benefits of pre-menarcheal gymnastics are retained after activity cessation. Pediatric Exercise Science 22, 21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerpella T.A., Dowthwaite J.N., Rosenbaum P.F. (2011) Sustained skeletal benefits from childhood mechanical loading. Osteoporosis International 22, 2205-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioen I., Lust E., De Henauw S., Moreno L.A., Jimenez-Pavon D. (2016) Associations between body composition and bone health in children and adolescents: a systematic review. Calcified Tissue International 99, 557-577. [DOI] [PubMed] [Google Scholar]
- Slemenda C.W., Peacock M., Hui S., Zhou L., Johnston C.C. (1997) Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. Journal of Bone and Mineral Research 12, 676-682. [DOI] [PubMed] [Google Scholar]
- Sööt T., Jürimäe T., Jürimäe J., Gapeyeva H., Pääsuke M. (2005) Relationship between leg bone mineral values and muscle strength in women with different physical activity. Journal of Bone and Mineral Metabolism 23, 401-406. [DOI] [PubMed] [Google Scholar]
- Sööt T., Jürimäe T., Jürimäe J. (2006) Relationships between bone mineral density, insulin-like growth factor-1 and sex hormones in young females with different physical activity. Journal of Sports Medicine and Physical Fitness 46, 293-297. [PubMed] [Google Scholar]
- Theodoropoulou A., Markou K.B., Vagenakis G.A., Benardot D., Leglise M., Kourounis G. (2005) Delayed but normally progressed puberty is more pronounced in artistic compared with rhythmic elite gymnasts due to the intensity of training. Journal of Clinical Endocrinology and Metabolism 90, 6022-6027. [DOI] [PubMed] [Google Scholar]
- Tournis S., Michopoulou E., Fatouros I.G., Paspati I., Michalopoulou M., Raptou O., Leontsini D., Avloniti A., Krekoukia M., Zouvelou V., Galanos A., Aggelousis N., Kambas A., Douroudos I., Lyritis G.P., Taxildaris K., Pappaioannou N. (2010) Effect of rhythmic gymnastics on volumetric bone mineral density and bone geometry in premenarcheal female athletes and controls. Journal of Clinical Endocrinology and Metabolism 95, 2755-2762. [DOI] [PubMed] [Google Scholar]
- Vaitkeviciute D., Lätt E., Mäestu J., Jürimäe T., Saar M., Purge P., Maasalu K., Jürimäe J. (2014) Physical activity and bone mineral accrual in boys with different body composition parameters during puberty: a longitudinal study. PloS One 9, e107759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkeviciute D., Lätt E., Mäestu J., Jürimäe T., Saar M., Purge P., Maasalu K., Jürimäe J. (2016) Longitudinal associations between bone and adipose tissue biochemical markers with bone mineralization in boys during puberty. BMC Pediatrics 16, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Rodriguez G., Dorado C., Ara I., Perez-Gomez J., Olmedillas H., Delgado-Guerra S., Calbet J.A. (2007) Artistic versus rhythmic gymnastics: effects on bone and muscle mass in young girls. International Journal of Sports Medicine 28, 386-393. [DOI] [PubMed] [Google Scholar]
- Võsoberg K., Tillmann V., Tamm A.L., Jürimäe T., Saar M., Maasalu K., Neissaar I., Lätt E., Jürimäe J. (2014) Adipocytokine and ghrelin levels in relation to body composition in rhythmic gymnasts entering into puberty: a three-year follow-up study. Pediatric Exercise Science 26, 477-484. [DOI] [PubMed] [Google Scholar]
- Võsoberg K., Tillmann V., Tamm A.L., Jürimäe T., Maasalu K., Jürimäe J. (2016) Adipocytokine and ghrelin levels in relation to bone mineral density in prepubertal rhythmic gymnasts entering puberty: a 3-year follow-up study. European Journal of Applied Physiology 116, 831-839. [DOI] [PubMed] [Google Scholar]
- Võsoberg K., Tillmann V., Tamm A.L., Maasalu K., Jürimäe J. (2017) Bone mineralization in rhythmic gymnasts entering puberty: associations with jumping performance and body composition variables. Journal of Sports Science and Medicine 16, 108-113. [PMC free article] [PubMed] [Google Scholar]
- Ward K.A., Roberts S.A., Adams J.E., Mugha M.Z. (2005) Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone 36, 1012-1018. [DOI] [PubMed] [Google Scholar]
- Weaver C.M., Gordon C.M., Janz K.F., Kalkwarf H.J., Lappe J.M., Lewis R., O'Karma M., Wallace T.C., Zemel B.S. (2016) The National Osteoporosis Foundations`s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporosis International 27, 1281-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Lombardi G., Jiao E., Banfi G. (2016) Effect of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sports Medicine 46, 1165-1182. [DOI] [PubMed] [Google Scholar]
- Zaman G., Cheng M.Z., Jessop H.L., White R., Lanyon L.E. (2000) Mechanical strain activates estrogen response elements in bone cells. Bone 27, 233-239. [DOI] [PubMed] [Google Scholar]
- Zanker C.L., Gannon L., Cooke C.B., Gu K.L., Oldroyd B., Truscott J.G. (2003) Differences in bone density, body composition, physical activity, and diet between child gymnasts and untrained children 7-8 years of age. Journal of Bone and Mineral Research 18, 1043-1050. [DOI] [PubMed] [Google Scholar]


