Table 3. Base screen for basicity and steric effects a .
| Base |
Δ-1
|
||
| % ee b | % yield of (S)-10 c | pKa d | |
| DBU | 34 | 20 | 24.33 |
| NEt3 | 72 | 55 | 18.82 |
| DIEA | 70 | 38 | 18.80 |
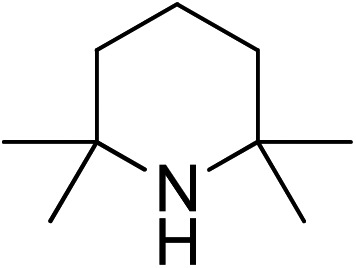
|
53 | 33 | 18.64 |
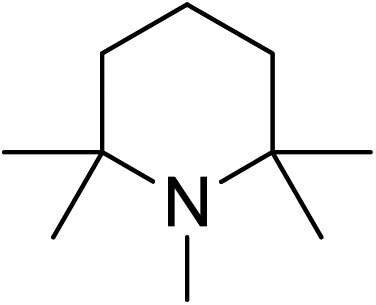
|
66 | 43 | 18.62 |
| DABCO | 66 | 44 | 18.29 |
| DMAP | 57 | 21 | 17.95 |
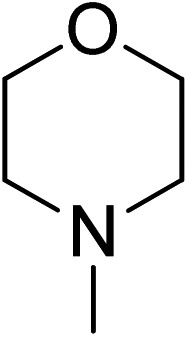
|
40 | 17 | 15.8 |
| Collidine | 10 | 8 | 14.77 |
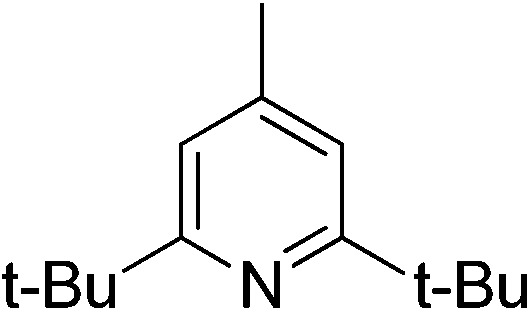
|
0 | 21 | 13–14 |
| TBAF | 20 | 61 | ? |
aAll reactions were performed using β-nitrostyrene 8 (0.34 mmol, 1 equiv.), diethylmalonate 9 (0.68 mmol, 2 equiv.), and base (0.034 mmol, 0.1 equiv.) in MeCN (1 mL) with 5 mol% catalyst (Δ-1) at room temperature for 24 h.
bDetermined by chiral HPLC analysis.
cIsolated yields.
dpKa of conjugate acid in acetonitrile.38
