Figure 1. Cellular consequences of expanded microsatellite repeats and repeat-associated non-ATG (RAN) translation.
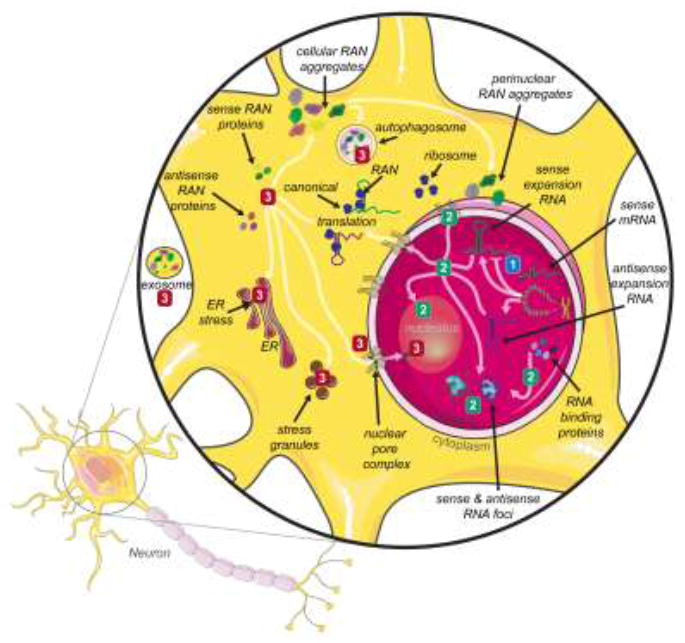
Although microsatellite repeats are expressed in a wide variety of cells, the majority of RAN proteins have been identified in the central nervous system (see box 1). While this generalized illustration utilizes a neuron, RAN proteins are found in a variety of CNS cell types (neurons, astrocytes, microglia, motorneurons, etc). Bidirectional transcription has been observed in a growing number of microsatellite repeat expansion diseases [96-99] resulting in the expression of both sense and antisense expansion transcripts. Three non-exclusive disease mechanisms have been proposed for the majority of microsatellite expansion diseases. (1) Loss-of-function (blue) – reduction in mRNA transcript resulting in reduced C9orf72 protein levels. The presence of expanded repeats in the DNA may result in epigenetic silencing and/or transcriptional inhibition that reduce the available levels of the gene’s canonical protein product. Protein loss-of-function has been proposed for the C9orf72 hexanucleotide repeats [35,39,100-102]. (2) RNA gain-of-function (green) – sequestration of RNA-binding proteins into nuclear foci by the expanded RNA repeats reduces the available RBP concentration and its normal cellular function. In DM1, CUG expansion RNAs sequester MBNL proteins from their normal splicing targets and MBNL loss-of-function leads to alternative splicing dysregulation and pathogenesis [37,52,59,62,63,103]. The expanded repeat transcript may also interact with, and alter the function of other cellular components, such as the interaction between G4C2 expansion RNA and proteins of the nuclear pore complex [59]. (3) Protein gain-of-function (red) – the production of up to six toxic RAN proteins from the sense and antisense expansion transcripts results in defects in cellular processes, including protein homeostasis. In C9-ALS/FTD, sense and antisense dipeptide RAN proteins have been shown to lead to nucleolar dysfunction, ER stress, stress granules, altered autophagy, transcellular transmission, disruption of nucleocytoplasmic defects and nuclear envelope defects [4,7,104,105].
