Abstract
Ovarian cancer is fairly unique in that ovarian carcinoma cells can detach and spread directly through peritoneal cavity. It has been unclear, however, how detached cancer cells survive in the peritoneum and form spheroid structure. We have recently reported that there is a strong correlation between Tumor-associated macrophages (TAMs)-associated spheroid and clinical pathology of ovarian cancer, and that TAMs promote spheroid formation and tumor growth at early stages of transcoelomic metastasis in orthotopic mouse models. We have established an in vitro spheroid formation assay using a 3D co-culture system in which mouse GFP+F4/80+CD206+ TAMs isolated from spheroids of ovarian cancer-bearing donor tomatolysM-cre mice were mixed with ID8 cells (TAM:ID8 at a ratio of 1:10) in medium containing 2% Matrigel and seeded onto the 24-well plate precoated with Matrigel. As transcoelomic metastasis is also associated with many other cancers such as pancreatic and colon cancers, TAM-mediated spheroid formation assay would provide a useful approach to define the molecular mechanism and therapeutic targets for ovarian cancer and other transcoelomic metastasis cancers.
Keywords: Ovarian cancer, Tumor-associated macrophage, 3D co-culture system, Spheroid formation, Transcoelomic metastasis
Background
Ovarian cancer (OC) is the second most common gynecological cancer and the leading cause of death in the United States ( Jemal et al., 2009 ; Siegel et al., 2012 ). The major reason for the poor prognosis of OC is intraperitoneal and pelvic extensive implantation metastasis, which is usually unable to be removed completely by surgery. The most widely ascribed explanation for the phenomenon of peritoneal metastasis is that tumor cells become detached from the primary tumor after extension into the peritoneal surface and are transported throughout the peritoneal cavity by peritoneal fluid before seeding intraperitoneally. It has been suggested that the process of transcoelomic metastasis could be divided into several steps: 1) cell detachment, survival and resistance of anoikis; 2) evasion of immunological surveillance; 3) epithelial-mesenchymal transition; 4) spheroid formation; 5) ascites formation; and 6) peritoneal implantation ( Tan et al., 2006 ; Peart et al., 2015 ; Rafehi et al., 2016 ). However, it remains unclear how free detached tumor cells survive in transcoelomic environment and form spheroids at initial steps of transcoelomic metastasis. Our recent study reveals that TAMs play an essential role in the survival and proliferation of free cells detached from the primary tumor in transcoelomic environment and spheroid formation at early stages of transcoelomic metastasis ( Yin et al., 2016 ).
One critical method in this study is an in vitro spheroid formation assay using a 3D co-culture system to determine how TAMs facilitate spheroid formation. In this assay, mouse GFP+F4/80+CD206+ TAMs isolated from spheroids of ovarian cancer-bearing donor tomatolysM-cre mice were mixed with ID8 cells (TAM:ID8 at a ratio of 1:10) in medium containing 2% Matrigel and seeded onto the 24-well plate precoated with Matrigel. Similarly, we use human CD14+ TAMs isolated from OC patients and human ovarian cancer SKOV3 cells. In this model, we detect spheroid formation at 48 h of co-culture (Figure 1).
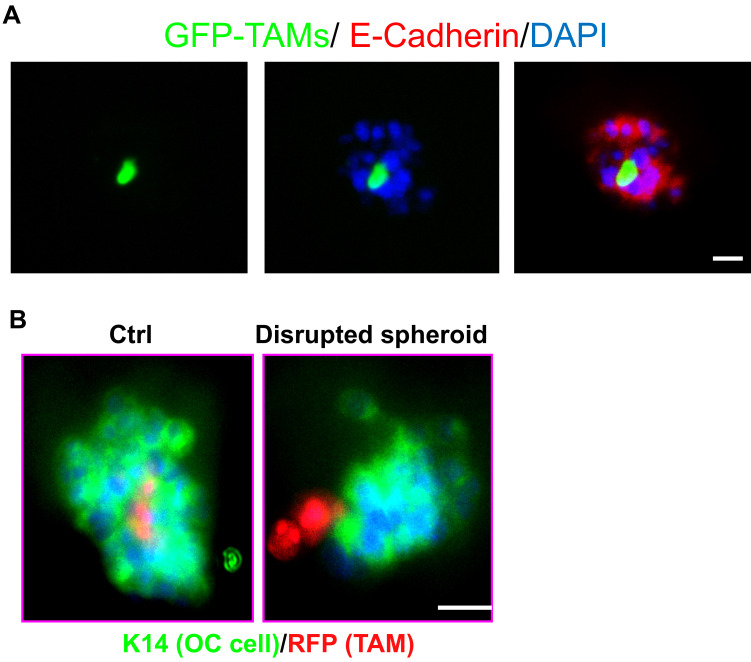
Figure 1. TAMs and OC cells in vitro 3D co-culture system were showed by Immunofluorescence.
A. TAMs and OC cells form spheroids in an in vitro 3D co-culture system. GFP+F4/80+CD206+ TAMs isolated from spheroids of ovarian cancer-bearing donor tomatolysM-cre mice and ID8 cells were co-cultured in the Matrigel-precoated 24-well plate for 48 h. The spheroids were subjected to immunofluorescent staining for E-cadherin for tumor cells. Images for GFP+ TAMs, E-Cadherin+ OC cells and DAPI for all cells in the spheroids are shown. B. Human TAMs were isolated and infected with lentivirus expressing RFP. RFP-expressing TAMs were incubated with SKOV3 human ovarian cancer cells followed by 3D co-culture for 72 h. Spheroids were immunostained with keratin-14. Scale bars = 10 μm.
Here, we summarize our detailed protocols for 3D spheroid formation assay.
Materials and Reagents
Pipette tips
15 cm Petri dish
10 ml serological pipette
50 ml sterile conical tube (Corning, Falcon®, catalog number: 352070)
18-gauge needle
10 ml syringe
FalconTM cell strainers,100 μm (Corning, Falcon®, catalog number: 352360)
1.5 ml microcentrifuge tubes
Greiner cellstar multiwall culture plates (24 wells, Greiner Bio One International, catalog number: 662102)
-
Cell lines: ID8 ovarian cancer cell line ( Yin et al., 2016 ) was a gift from Jack Lawler and Carmelo Nucera at Beth Israel Deaconess Medical Center (Harvard Medical School, Boston, Massachusetts, USA).
Note: ID8 cells are mouse epithelial OC line derived from C57BL/6 background; Passage under 30 and less than 1 week culture before injections.
C57BL/6 mice, female, age: 8 weeks
Tomato reporter transgenic mice: ID8 OC cells were labeled by stably expressing mCherry fluorescence protein while LysMCre mice crossed to the tomato reporter mT/mG
Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 10567014)
D-glucose
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 26140079)
Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140)
0.25% Trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
Ketamine
Bovine serum albumin (BSA) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: B14)
Collagenase (Thermo Fisher Scientific, GibcoTM, catalog number: 17104019)
F4/80 monoclonal antibody, APC conjugate for flow cytometry (Thermo Fisher Scientific, Invitrogen, catalog number: MF48005)
PE anti-mouse CD206 (MMR) antibody (BioLegend, catalog number: 141705)
DAPI (Vector Laboratories, catalog number: H-1200)
Anti-mouse E-Cadherin antibody (BD, PharmingenTM, catalog number: 610404)
Donkey anti-mouse (Alexa Flour 594) (Thermo Fisher Scientific, InvitrogenTM, catalog number: A-21203)
Live cell tracker CMFDA (Thermo Fisher Scientific, InvitrogenTM, catalog number: C2925)
EGFP (abm, catalog number: LV011-a)
Na2HPO4
NaCl
KH2PO4
KCl
Corning Matrigel (Basement membrane matrix, Corning, catalog number: 356234)
Tween 20 (Sigma-Aldrich, catalog number: P7949-500ML)
Paraformaldehyde (Sigma-Aldrich, catalog number: 16005)
PBS (see Recipes)
PBST (see Recipes)
AC buffer (0.5% BSA) (see Recipes)
2% Matrigel (see Recipes)
3.7% paraformaldehyde (see Recipes)
Equipment
Safety cabinet
Pipette-aid
Centrifuge with swinging-bucket rotor and adaptors for 50-ml conical tubes
Water bath set at 37 °C
Humidified cell culture incubator set to 37 °C and 5% CO2
Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss, model: Axiovert 200)
Upright microscope with 10x objective
Cell sorter and the scale (BD, model: FACSAriaTM II); sorting at a rate of 80,000 cell/h
Software
Quantitation of the average distance between the geometrical center of nuclei of adjacent cells can be measured using Openlab3 software (Improvision, Lexington, MA) or other commercially available image analysis software
SAS software (version 9.1.4, SAS Institute, Cary, NC)
Procedure
Note: All animal studies were approved by the Institutional Animal Care and Use Committee of Yale University.
-
Macrophage isolation and labeling
Cross Lysozyme Cre mice with tomato reporter C57BL/6 background mice and isolate EGFP-positive macrophages from peritoneal cavity by FACS sorting ( Yin et al., 2016 ).
As a positive control for spheroids, ID8 cells (1 x 106/ml) are injected into the abdominal cavity of Lysozyme Cre C57BL/6 mice (Age: 8 weeks) in 100 μl of DMEM. Sacrifice the mice after nine weeks according to procedures approved by Yale’s Institutional Animal Care and Use Committee, and analyze and sort the primary mouse tumor-associated macrophages (TAMs-GFP) using FACS ( Yin et al., 2016 ).
-
Preparation of ID8 cell cultures
-
Expand the ID8 mouse ovarian cancer cells (Number of starting cells: 1 x 106 ) in DMEM (4.5 g/L D-glucose, 10% FBS, 100 IU penicillin and 100 μg/ml streptomycin) on a 15 cm Petri dish and culture ID8 cells until reaching 70-80% confluence.
Preparation: Prepare a safety cabinet. Switch on and allow the cabinet to reach working airflow pressure for approximately 15 min before use.
Warm medium. To avoid unnecessary stress to the cells, pre-equilibrate the medium in the incubator. If you are going to passage adherent cells, also thaw and warm an aliquot of trypsin.
Checking cells: Cells should be checked microscopically daily to ensure they are healthy and growing as expected. Attached cells should be mainly attached to the bottom of the Petri dish, round and plump or elongated in shape and refracting light around their membrane.
Upon reaching 70-80% confluence, aspirate the culture medium from the dish and add 10 ml of fresh DMEM.
Harvest the ID8 cells by washing them from the Petri dish with the DMEM (10 ml) using a pipette-aid and 10 ml serological pipette.
Transfer the ID8 cell suspension from the plate to a 50 ml conical tube, and pellet the cells by centrifugation at 200 × g for 4 min (room temperature).
Suspend the ID8 cell in DMEM and count the cells and prepare a suspension of the ID8 in DMEM at a density of 1 x 106 cells per ml and ID8 cells should be kept on ice for injection.
-
-
ID8 tumor implantation and metastasis model
Pathogen-free C57BL/6 background mice are purchased from THE JACKSON LABORATORY.
ID8 cells (1 x 106/ml in 100 μl of DMEM) will be injected into the abdominal cavity of C57BL/6 background mice.
Mice body weight gains will be measured weekly.
The mice will be sacrificed at nine weeks prior to obvious signs of distress, pain or death.
-
Ascitic fluid volume and tumor weight will be measured, and the tumor spheroids will be analyzed by histology and FACS.
Ascitic fluid may begin to build up within 8-10 weeks following the injection of the ID8 cells. Tap the fluid when the mouse is noticeably large, but before the mouse has difficulty moving. Carefully withdraw as much fluid as possible with an 18-gauge needle attached to 10 ml syringe.
Note: Sedating the mouse will make the collection of the ascitics fluid easier: ketamine 2 ng/mouse.
-
Collect TAMs from tumor spheroids (Figure 3)
Collect tumor spheroids from 3 ml ascitic fluid by using FalconTM cell strainers.
Transfer the tumor spheroids to a 50 ml conical centrifuge tube, add wash medium (0.5% BSA with PBS 10 ml) for 2 min and discard it, and then add wash medium, repeat this washing step 3 times.
At the end of the washing cells, centrifuge the cell suspension at 200 × g for 3 min (room temperature).
Discard the wash medium and add 10 ml 500 U/ml collagenase in DMEM solution to the tube containing the tumor spheroids.
-
Incubate the tube at 37 °C for 1 h, and swirl the tube vigorously every 15 min. At the end of the incubation period, spheroid should be almost completely digested and no longer visible.
Note: If spheroids are still visible after 1 h, continue incubation, checking every 15 min, until spheroids are no longer visible (do not exceed 2.5 h).
At the end of the collagenase digestion, centrifuge the cell suspension at 200 × g for 10 min (room temperature).
Remove the supernatant from the tube carefully without dislodging the pellet.
-
Add 2 ml FACS buffer (0.5% BSA with PBS) to the 50 ml tube and suspend the cell pellet, and then transfer the suspension to a FACS tube through filters (Corning Falcon 5 ml round bottom tube with cell strainer snap cap, A 35 μm nylon mesh).
Note: It is necessary to obtain a single cell suspension.
Centrifuge the cell suspension again at 200 × g for 10 min (room temperature).
Remove the supernatant from the tube carefully without dislodging the pellet.
Resuspend the pellet in 500 μl FACS buffer.
Add 2.5 μl F4/80 (APC, 0.2 mg/ml) and 5 μl CD206 (PE, 0.2mg/ml) antibody to stain TAMs.
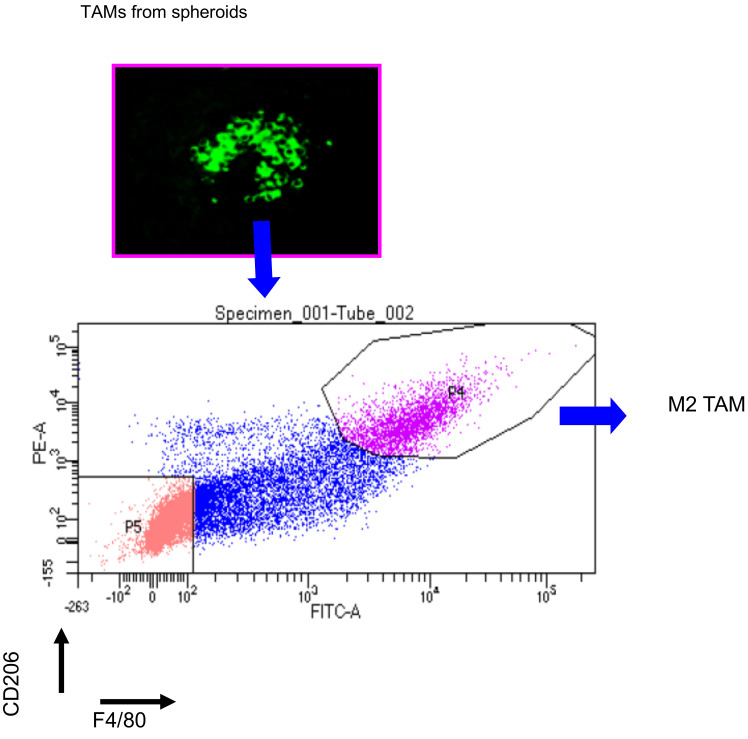
Sort GFP+ F4/80+ and CD206+ TAMs through FACS analysis (Figure 2).
-
Precoat 24-well plate with Matrigel
A 3D culture model was examined to determine the suitable cell culture system for our study.
In the 3D-base model, a 24-well plate is precoated with Matrigel as basement membrane by adding 160 μl of Matrigel to each well.
Incubate at 37 °C for 30 min to let the Matrigel solidify.
Plate the cells (TAMs from step D7, ID8 cells or SKOV-3) onto the gel in 1 ml regular medium containing 2% Matrigel (3D-base and embedded, containing laminin as a major component, collagen type IV, heparin sulfate proteoglycan, entactin, and other minor components).
-
3D co-culture system of TAM and ID8 cells
Isolate mouse GFP+ F4/80+ and CD206+ TAMs by FACS sorting from spheroids of ovarian cancer-bearing donor tomatolysM-cre mice.
Precoat the 24-well plates with Matrigel as described above.
Seed the mixtures of TAMs and ID8 cells (at a ratio of 1:10 but with a fixed total cell number as 40,000 cells/well) directly onto the Matrigel-precoated 24-well plate with 300 μl DMEMs in each well.
-
Incubate the cells were incubated at 37 °C for up to 48 h to allow the aggregates spheroids to form.
Note: The wells without cells but containing medium are used as negative control.
-
E-Cadherin staining in ID8 cells by immunofluorescent method
Gently wash the wells 2 times each with 500 μl PBS to remove DMEM.
After PBS removal, add 300 μl of buffered paraformaldehyde (3.7% paraformaldehyde, 10 nM, pH 7.4) for 10 min.
Wash the wells 2 times with PBS for removing the PFA and add 100 μl E-Cadherin antibody (1:200 with 1% BSA) to incubate at 4 °C overnight (staining protocol see Yin et al., 2016 ).
On the second day wash spheroids 3 times with 500 μl PBST for about 15 min/time.
Add 100 μl PE anti-mouse second antibody (1:500 with 1% BSA) into the 24-well plate with spheroids and incubate for 1-h at RT.
Capture fluorescent microscopic images by using Openlab 3 software (Improvision, Lexington, MA) under Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss MicroImaging; Thornwood, NY). The captured images are used for analyzing the cell morphology.
Figure 3. The steps of collecting TAMs from spheroids.
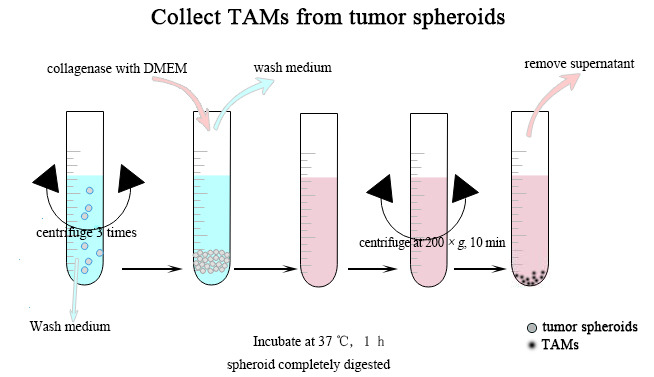
Figure 2. FACS sorting of M2 TAMs.
Spheroids were collected from ovarian cancer-bearing donor mice and dispersed to single cells. Cells labeled with anti-F4/80 and anti-CD206 were isolated by FACS sorting on FACSAriaII (BD Biosciences).
Data analysis
Spheroids were visualized directly under a fluorescence microscope or were subjected to Line were subjected to immunostaining with anti-CD68, anti-K14 and DAPI followed by confocal imaging (see Figure 1).
The number (per well) and size (area) of spheroids at 48 h were quantified.
Statistical analyses: The differences of results of spheroid formation were analyzed by Student’s t-test. Statistical analyses in this study were performed using SAS software (version 9.1.4, SAS Institute, Cary, NC). All statistical tests were two-tailed, and P-values less than 0.05 were considered statistically significant. All data are presented as means ± SEM, n = 5, *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Notes
For Matrigel preparation, freeze pipette tips to better handle Matrigel (which should be used at 4 °C).
Alternatively, mouse peritoneal macrophages from C57BL/6 background mice were isolated followed by pre-staining with live cell tracker CMFDA ( Qin et al., 2014 ) or by transduction with lentivirus expressing EGFP as we have shown for vascular endothelial cells by FACS sorting ( Zhou et al., 2016 ).
Euthanasia: Mice were euthanized with an overdose of ≥ 100 mg/kg bw pentobarbital and exsanguinated.
SKOV3 human ovarian cancer cells: (human ovarian adenocarcinoma cell line) were obtained from ATCC. SKOV3 cells are resistant to tumor necrosis factor and several cytotoxic drugs, including diphtheria toxin, cisplatinum, and adriamycin.
Recipes
-
PBS
8 mM Na2HPO4
136 mM NaCl
2 mM KH2PO4
2.6 mM KCl
-
PBST
500 ml 1x PBS
1 ml Tween-20
-
AC buffer (0.5% BSA)
Add 50 mg BSA into 10 ml PBS
-
Regular medium
Add 50 ml fetal bovine serum and 5 ml penicillin and streptomycin into 500 ml DMEM medium
-
2% Matrigel
Add 200 μl Matrigel into 10 ml regular medium
Note: 2% Matrigel contains 8-12 mg/ml protein which includes laminin (major component), collagen type IV, heparin sulfate proteoglycan, entactin, and other minor components. Addition of Collagen type IV to the gel increases polymerization.
-
3.7% paraformaldehyde
Add 37 mg paraformaldehyde into 1 ml PBS
Acknowledgments
Conflict of interest disclosure: None.
This work was partly supported by National Key Research and Development Program of China (2016YFC1300600), National Natural Science Foundation of China (No. 91539110), and Scientific Grants of Guangdong (Nos. 2015B020225002 and 2015A050502018) to WM, R01 HL109420 and R01 HL115148, and CT Stem Cell Innovation Award (Established Investigator Grant) 14-SCB-YALE-17 to WM.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Jemal A., Siegel R., Ward E., Hao Y., Xu J. and Thun M. J.(2009). Cancer statistics, 2009. CA Cancer J Clin 59(4): 225-249. [DOI] [PubMed] [Google Scholar]
- 2. Peart T., Ramos Valdes Y., Correa R. J., Fazio E., Bertrand M., McGee J., Prefontaine M., Sugimoto A., DiMattia G. E. and Shepherd T. G.(2015). Intact LKB1 activity is required for survival of dormant ovarian cancer spheroids. Oncotarget 6(26): 22424-22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin L., Huang Q., Zhang H., Liu R., Tellides G., Min W. and Yu L.(2014). SOCS1 prevents graft arteriosclerosis by preserving endothelial cell function. J Am Coll Cardiol 63(1): 21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rafehi S., Ramos Valdes Y., Bertrand M., McGee J., Prefontaine M., Sugimoto A., DiMattia G. E. and Shepherd T. G.(2016). TGFβ signaling regulates epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr Relat Cancer 23(3): 147-159. [DOI] [PubMed] [Google Scholar]
- 5. Siegel R., Naishadham D. and Jemal A.(2012). Cancer statistics, 2012. CA Cancer J Clin 62(1):10-29. [DOI] [PubMed] [Google Scholar]
- 6. Tan D. S., Agarwal R. and Kaye S. B.(2006). Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol 7(11): 925-934. [DOI] [PubMed] [Google Scholar]
- 7. Yin M., Li X., Tan S., Zhou H. J., Ji W., Bellone S., Xu X., Zhang H., Santin A. D., Lou G. and Min W.(2016). Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest 126(11): 4157-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou H. J., Qin L., Zhang H., Tang W., Ji W., He Y., Liang X., Wang Z., Yuan Q., Vortmeyer A., Toomre D., Fuh G., Yan M., Kluger M. S., Wu D. and Min W.(2016). Endothelial exocytosis of angiopoietin-2 resulting from CCM3-deficiency contributes to the progression of cerebral cavernous malformation. Nat Med 22(9): 1033-42. [DOI] [PMC free article] [PubMed] [Google Scholar]