Fig 1. Most, but not all mtLSU proteins are essential for mitochondrial protein synthesis and respiration. See also Figure S1.
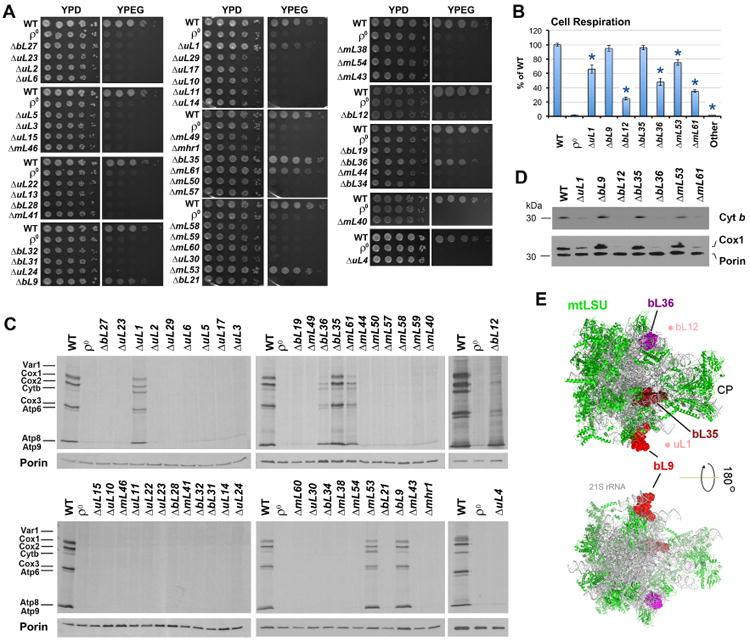
A. Serial dilution growth test of WT (W303I0) and MRP deletion mutant strains in fermentable solid media containing glucose (YPD) and non-fermentable, respiratory media containing ethanol and glycerol (YPEG).
B. Endogenous cell respiration for WT and MRP mutant strains measured polarographically in cells growing in synthetic media containing 2% galactose and 0.5% glucose. “Other” refers to the rest strains; full data is presented in Supplemental Table S2. n=3 biological replicates. Data are mean ± SD. *p<0.05.
C. Metabolic labeling with 35S-methionine of newly synthesized mitochondrial translation products in the presence of cycloheximide to inhibit cytoplasmic protein synthesis. Immunoblotting for Porin was used as a loading control.
D. Immunoblot analyses of Cox1 and cytochrome b (Cyt b) steady-state levels in the indicated strains, using Porin as a loading control.
E. Location of non-essential proteins in the mtLSU are shown on structures of the S. cerevisiae mitochondrial ribosome (PDB 5mrc) (Desai et al., 2017). Solvent-facing (upper panel) and interface-facing (lower panel) views are presented. Proteins bL36 (magenta), bL35 (firebrick) and bL9 (red) are shown as surface and the rest of proteins (green) are shown as ribbon diagrams. The 21S rRNA is shown in grey. The densities for uL1 and bL12 were not identified in the structure but the area where they localize (based on the structure of bacterial ribosomes) is indicated by light reddish dots for orientation purposes. CP, central protuberance.
