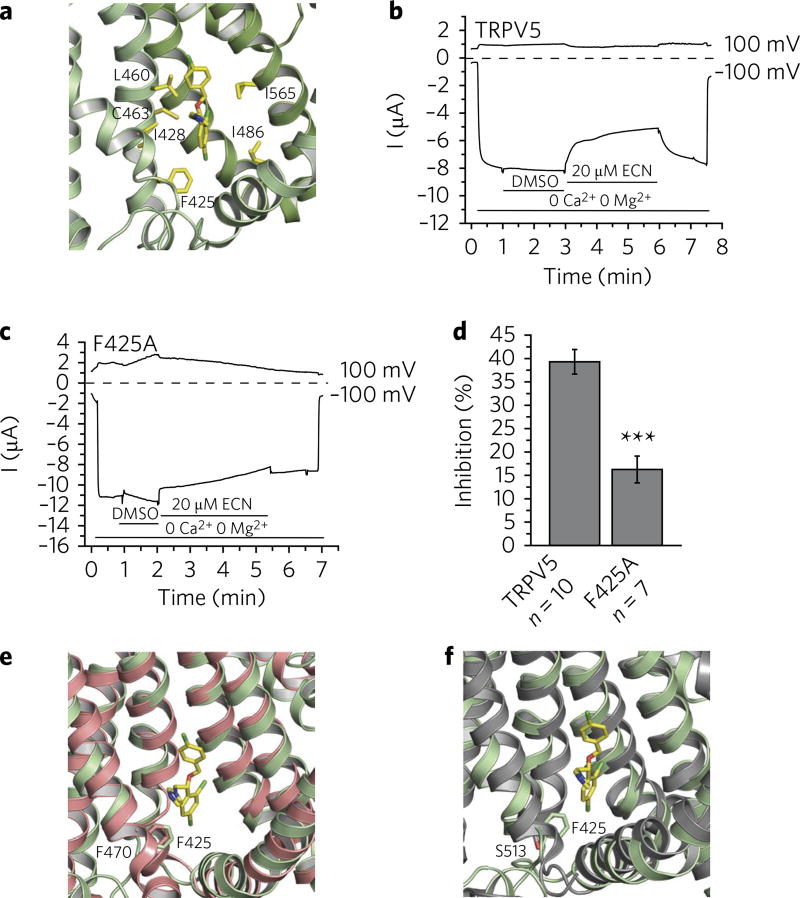
Fig. 2. Econazole inhibition of TRPV5.
a, Zoomed-in view of the econazole-binding pocket. (R)-econazole is represented as yellow sticks. Residues that stayed within 4.0 Å from at least three of the four econazole molecules during the MD simulations with either enantiomeric form are labeled and shown in yellow. b, A representative TEVC trace of wild-type rabbit TRPV5 in Xenopus oocytes (n = 10 individual traces) at 100 mV and −100 mV in the presence of 2.2% DMSO (solvent) and 20 µM econazole (ECN), indicated by horizontal lines. TRPV5 currents under basal conditions are largely blocked by the 1 mM Mg2+ and trace amounts of Ca2+ in the bath solution. Monovalent currents are initiated by removing Mg2+ and chelating Ca2+ with 1 mM EGTA. c, A representative TEVC trace of the F425A mutant rabbit TRPV5 in Xenopus oocytes (n = 7 individual traces) at 100 and −100 mV in the presence of 2.2% DMSO (solvent) and 20 µM econazole (ECN), indicated by horizontal lines. TRPV5 currents under basal conditions are largely blocked by the 1 mM Mg2+ and trace amounts of Ca2+ in the bath solution. Monovalent currents are initiated by removing Mg2+ and chelating Ca2+ with 1 mM EGTA. d, Statistical summary (% inhibition) at −100 mV, ***P < 0.001, two-sample t test. Data shown are mean ± s.e.m. e,f, The econazole binding-pocket aligned to the ligand-binding pockets of TRPV2 (PDB 5AN8) (e) and TRPV1 (PDB 3J5R) (f). Residues of interest are labeled and shown as sticks.